Figure 2.
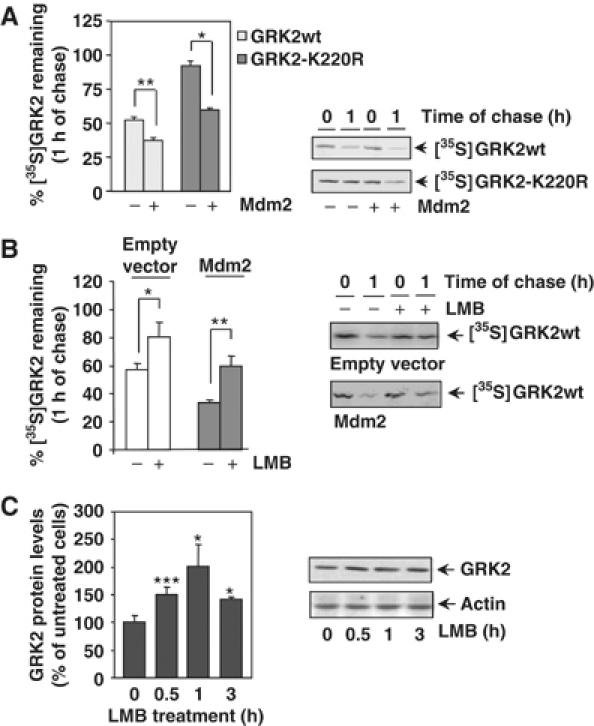
(A) Mdm2 increases degradation of GRK2 and of a slow-turnover GRK2 mutant. HEK-293 cells were transiently transfected with wild-type GRK2 or the GRK2-K220R mutant in the presence or absence of Mdm2, and kinase turnover assessed by pulse–chase experiments as described in Materials and methods. 35S-labeled proteins immunoprecipitated with the anti-GRK2 antibody AbFP2 were resolved by SDS–PAGE followed by fluorography and densitometry. 35S-labeled GRK2 band densities were then normalized to total GRK2 present in the immunoprecipitates, as determined by Western blot analysis. Data are mean±s.e.m. of 3–4 independent experiments performed in duplicate, *P<0.05, **P<0.01. A representative gel autoradiography is shown. (B, C) Effect of LMB treatment on GRK2 turnover. HEK-293 cells transfected with GRK2 and Mdm2 or an empty vector were treated with LMB or vehicle before and during pulse–chase experiments as detailed in Materials and methods, and GRK2 turnover determined as in previous figures. Data are the mean±s.e.m. of three independent experiments performed in duplicate, *P<0.05, **P<0.01. Representative fluorographs are shown. In panel C, endogenous GRK2 expression levels were determined by immunoblot analysis in lysates from MCF7 cells treated for different times with LMB. Data are corrected for actin expression and depicted as percentage of control. *P<0.05, ***P<0.001. A representative blot is shown.
