Figure 3.
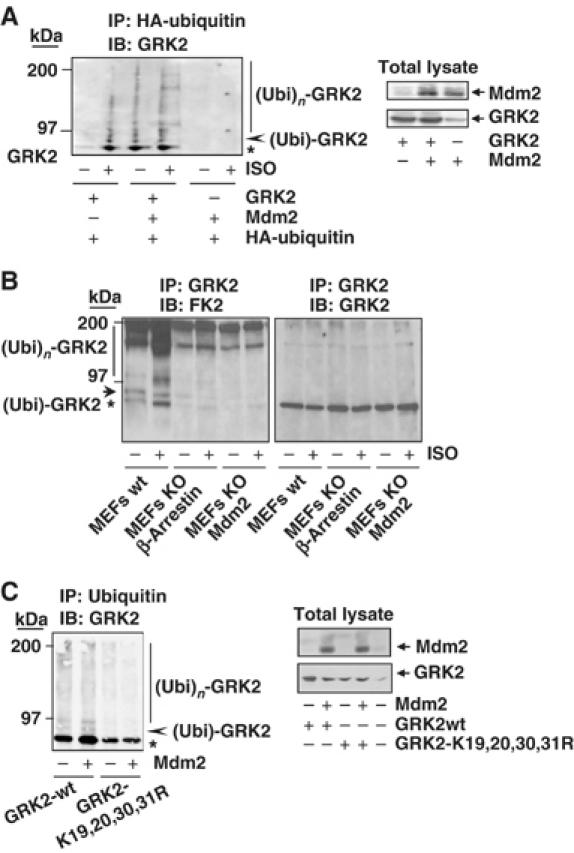
Mdm2 promotes GRK2 ubiquitination. (A) Wild-type GRK2, HA-ubiquitin and β2AR expression plasmids were transfected together with Mdm2 or an empty vector into HEK-293 cells. After 2 h of serum starving, cells were incubated with or without 10 μM isoproterenol for 15 min and lysed for ubiquitination assays as described in Materials and methods. The different GRK2 species present in the ubiquitin immunoprecipitates are indicated. Asterisk and arrowhead indicate mono- and bi-ubiquitinated GRK2 forms (see text for details). Blots depicting expression levels for Mdm2 and GRK2 in total lysates are shown. (B) Both basal and agonist-stimulated ubiquitination of endogenous GRK2 are blocked in either Mdm2- or β-arrestin-deficient cells. ∼50 × 106 wild-type MEFs or MEFs devoid of Mdm2 or β-arrestin proteins were serum-starved and challenged or not with 10 μM isoproterenol for 15 min. Cells were processed as indicated above and ubiquitinated GRK2 species were analyzed by immunoprecipitation of endogenous GRK2 with a specific monoclonal anti-GRK2 antibody (c5/1.1) and detection of endogenous ubiquitin conjugates with anti-ubiquitin antibody FK2. Membranes were stripped and immunoblotted (right panel) with a specific polyclonal anti-GRK2 antibody (C-15) to confirm equal GRK2 loading in each condition. (C) Mutation of critical lysine residues in the N-terminus of GRK2 impairs kinase ubiquitination in the presence of Mdm2. Wild-type GRK2 or the GRK2- K19,20,30,31R mutant was cotransfected with ubiquitin plasmids in the presence or absence of Mdm2 into HEK-293 cells and the kinase ubiquitination pattern was analyzed as above. Expression levels of GRK2 and Mdm2 were assessed in lysates by Western blot analysis. Gels in all panels are representative of 2–3 independent experiments.
