Figure 1.
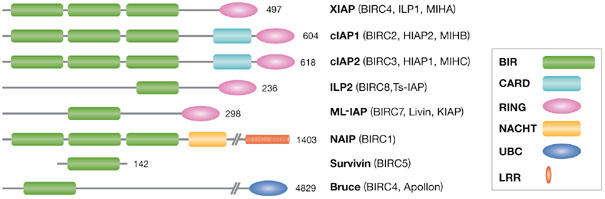
Schematic representation of the human inhibitor of apoptosis protein family. In addition to at least one baculoviral IAP repeat (BIR) domain, most IAPs have other distinct functional domains. The really interesting new gene (RING) domain found in many IAPs is an E3 ligase that presumably directs targets to the ubiquitin-proteasome degradation system. Caspase-recruitment domains (CARDs) can mediate homotypic protein–protein interactions, although the binding partners for the cellular IAP (cIAP) CARDs have not yet been elucidated. Bruce has a ubiquitin-conjugation (UBC) domain that is found in many ubiquitin-conjugating enzymes. The NACHT domain of neuronal apoptosis-inhibitory protein (NAIP) resembles a nucleotide-oligomerization domain related to the AAA+ NTPases, whereas the leucine-rich repeats (LRRs) are similar to those of the Toll-like receptors that function as pathogen sensors. BIRC, baculoviral IAP-repeat-containing; HIAP, human IAP; IAP, inhibitor of apoptosis protein; ILP, IAP-like protein; KIAP, kidney IAP; MIH, mammalian IAP homologue; ML-IAP, melanoma IAP; NACHT, domain found in NAIP, CIITA, HET-E and TP-1; Ts-IAP, testicular IAP; XIAP, X-linked IAP.
