Figure 9.
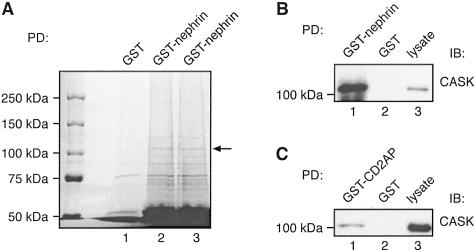
A: Identification of CASK in GST-nephrin tail pull-downs by mass spectrometry. Twenty μg of GST-nephrin tail or GST alone were incubated with 400 μg of glomerular lysate as in Figure 6. Bound proteins were resolved by 6% SDS-PAGE and stained with GelCode Blue. A prominent ∼110-kd band (arrow) present in the GST-nephrin tail (lanes 2 and 3) but not GST (lane 1) pull-downs was excised, digested with trypsin, and identified by mass spectrometry as CASK. To upscale the reactions, six parallel pull-downs with GST-nephrin tail and three with GST alone were performed, and three parallel samples were combined per lane. The ∼110-kd bands observed in GST-nephrin tail pull-downs in lanes 2 and 3 were combined for analysis by mass spectrometry. B and C: The presence of CASK in GST-nephrin tail (B) and CD2AP (C) pull-downs was confirmed by immunoblotting. Twenty μg of GST-nephrin tail, GST-CD2AP, or GST alone were incubated with 400 μg of glomerular lysate as in Figure 8, and immunoblotted with anti-CASK IgG. CASK binds to GST-nephrin tail (lane 1 in B) and GST-CD2AP (lane 1 in C) but not to GST alone (lane 2). Glomerular lysate (10 μg) is included as control (lane 3).