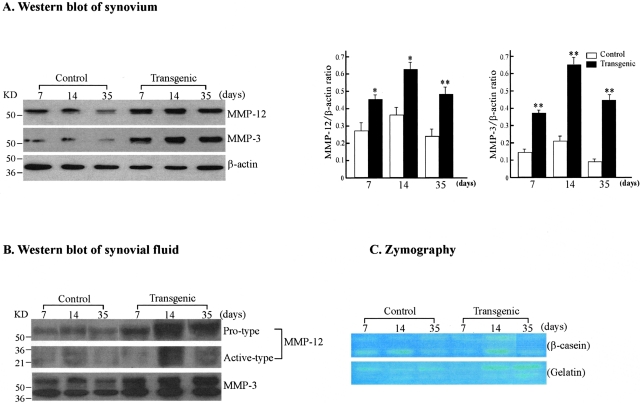
Figure 7-4264.
Immunoblotting and zymographic analyses of MMP-12 and MMP-3 in the synovium and synovial fluid. A: Western blot analysis of MMP-12 and MMP-3 expression in the synovium. The synovial tissue collected from control and Tg arthritic rabbits at 7, 14, and 35 days was homogenized. Ten μg of the crude proteins from each sample were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions, and probed with Ab against either MMP-12 (top) or MMP-3 (middle). The same membrane was reprobed with mAb against β-actin to verify that equal amounts of proteins were loaded (bottom). The relative levels of MMP-12 and MMP-3 was quantitated by calculating the optical density (OD) of each signal intensity on the films using a densitometer (Bio-Rad) and normalized relative to the amount of β-actin protein (right). The analysis was performed in triplicate and values are expressed as the mean ± SE. *, P < 0.05 or **, P < 0.001 versus control. B: Western blot analysis of MMP-12 and MMP-3 expression in the synovial fluid from rabbits with inflammatory arthritis. Ten μg of proteins of the synovial fluid from control and Tg arthritic rabbits at 7, 14, and 35 days were analyzed as above. Note that synovial fluid from Tg rabbits contained more MMP-12 and MMP-3 proteins (both pro- and active type) than that from control rabbits. C: Zymographic analysis of MMP activity in the synovial fluid. To assess the enzymatic activity of MMPs, zymography with β-casein (top) and gelatin (bottom) as substrates was performed. The caseinolytic activity present at 54 and 45 kd in Tg rabbit synovial fluid was markedly increased compared to that in the control (top) at 14 days. The gelatinolytic activity in Tg rabbit synovial fluid tended to be stronger than that in controls at 14 and 35 days.