Fig. 1.
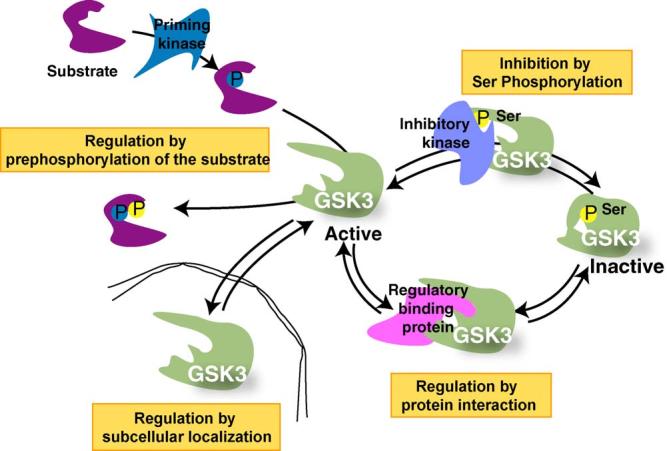
Mechanisms that regulate the actions of GSK3. Four mechanisms act in concert to regulate the phosphorylation of substrates by GSK3. Substrate phosphorylation by GSK3 is limited by the activity of the priming kinase which prepares the substrate for GSK3 because GSK3 most often phosphorylates primed substrates that are prephosphorylated four residues C-terminal to the GSK3 phosphorylation site. A major mechanism for inhibiting the activity of GSK3 is via serine-phosphorylation, so activity is inhibited when serine-9 of GSK3β or serine-21 of GSK3a is phosphorylated. Conversely, the activity of GSK3 is optimal when phosphorylated on tyrosine-216 of GSK3β or tyrosine-279 of GSK3a (not shown). When the substrate is prephosphorylated and GSK3 is active, with the regulatory serine dephosphorylated, two spatial restrictions also contribute to regulating the actions of GSK3, its subcellular localization and its association with other proteins in regulatory complexes. GSK3 is considered to be largely a cytosolic enzyme, but it is also associated with, or internalized in, subcellular compartments such as the nucleus, mitochondria, and growth cones, so dynamic regulation of the subcellular localization of GSK3 can regulate its access to substrates within subcellular compartments. Besides this gross cellular distribution of GSK3, its distribution in the cell is constrained by its propensity to be associated in protein complexes which provides an important mechanism for regulating its phosphorylation of specific substrates that are colocalized in such complexes. Thus, substrate-specific regulation of phosphorylation by GSK3 is achieved by regulation of the priming kinase activity, phosphorylation of GSK3, the subcellular localization of GSK3, and assembly of GSK3 in protein complexes.
