Abstract
Male-biased dispersal is a common trait in mammals, including carnivores, but its genetic consequences at the population level have been rarely considered for solitary species. We used long-term genetic data from cougars (Puma concolor) in and around Yellowstone National Park to test predictions based on differences in dispersal behaviour among males and females. Consistent with frequent long-distance dispersal of males, we found support for our prediction of less than expected allele sharing in pair-wise comparisons. In contrast, female residents present at the same time and females separated by few generations failed to share more alleles than expected, contrary to our predictions based on limited female dispersal. However, we find that genetic contributions of females with higher reproductive success were still noticeable in subsequent generations, consistent with female offspring showing fidelity to their natal area. These results highlight the importance of male dispersal for inbreeding avoidance, but do not indicate that short-distance dispersal or philopatry in female cougars results in spatial clustering of related individuals.
Keywords: emigration, immigration, microsatellites, philopatry, Puma concolor, relatedness
1. Introduction
Sex-biased dispersal, the greater tendency of one sex to leave the natal area, has been of interest to biologists for decades because of its effects on life-history evolution and genetic population structure (Greenwood 1980; Goudet et al. 2002). In most social carnivores, females remain with their natal group, which results in clusters of highly related individuals (Packer et al. 1991; Gompper et al. 1998; Van Horn et al. 2004). Such high relatedness forms the basis of kin selection and cooperative behaviour, but relies on male dispersal as the predominant means of inbreeding avoidance (Pusey 1987; Perrin & Goudet 2001).
Male-biased dispersal is also common in solitary carnivores (Waser & Jones 1983), but few studies have examined the population genetic consequences of this trait. For raccoons (Procyon lotor), Ratnayeke et al. (2002) confirmed the corollary expectation that local females are genetically more similar than males. If females remain close to their natal site (i.e. exhibit philopatry) and form local matrilineages (Rogers 1987; Kelly 2001; Logan & Sweanor 2002), one would further expect that females in a population may often be related. This has significant implications for evolutionary questions regarding resource sharing or competition among close kin. However, it has yet to be established whether limited female dispersal in solitary carnivores indeed results in local clusters of related females (i.e. sharing more alleles than expected by chance; Ratnayeke et al. 2002). In addition, one would predict that if long-distance dispersal is the norm in males, this should result in an opposite pattern of particularly low relatedness among local males. Here, we use long-term data from cougars in the northern Yellowstone ecosystem (NYE) to further examine these issues. Cougars are a good species to study these questions because they are solitary, territorial, and show long dispersal distances in males, whereas females are frequently philopatric (Logan & Sweanor 2002).
Given the pronounced differences in dispersal behaviour among female and male cougars, we wanted to know whether this would result in predictable genetic patterns for each sex within a population, both during one time period and over time. We therefore sought to test the following predictions:
resident females sampled during the same time share more alleles than expected by chance (i.e. are related) due to philopatry whereas males share fewer than expected due to long-distance dispersal.
-
The formation of matrilineages causes resident females to share more alleles with resident females from previous generations than expected, whereas males, which are usually born outside the study population, share fewer alleles with these females.
Because for prediction (ii), average relatedness may not be as informative if reproductive success is biased towards few individuals (Kelly 2001), we also made the following prediction:
alleles of reproductively more successful females are more frequently represented in subsequent generations of females sampled in the study area, but no such relationship exists for males.
Testing these predictions for NYE cougars should add to our understanding of the role of dispersal in gregarious and solitary mammals and provides an example for the usefulness of molecular tools for delineating differences in dispersal patterns between the sexes (Goudet et al. 2002).
2. Material and methods
(a) Samples
Samples were collected as part of a long-term research project on cougar population biology in NYE. The area, around 4300 km2 in size, incorporated the northern part of Yellowstone National Park and surrounding private and public land and had an estimated minimum resident cougar population of 12–18 individuals. Initial sampling (phase I) was conducted from 1988 to 1995 (Murphy 1998), followed by a second sampling period (phase II) from 1998 to 2003. Blood samples of 50 (phase I) and 49 cougars (phase II) were collected (table 1), representing more than 90% of the residents present during either study phase. Residents were defined based on radio tracking data as individuals of reproductive age that occupied a home rage within the study area for at least one breeding season.
Table 1.
Number of cougars genetically sampled.
| phase I (1988–1995) | phase II (1998–2003) | total | |
|---|---|---|---|
| Northern Yellowstone ecosystem: | |||
| resident females | 13 | 13 | 26 |
| resident males | 7 | 6 | 13 |
| kittens and non-residents | 30 | 30 | 60 |
| greater Yellowstone area | — | 29 | 29 |
| total | 50 | 78 | 128 |
Because hunting was prohibited in the park and overall adult mortality was relatively low in the study area, residents were usually present for several years. Two females that were born and became mature during phase I, were still present during phase II, but were only included as phase I residents.
(b) Relatedness
DNA extracted from blood samples was used to amplify 11 microsatellite loci (see electronic supplemental material). Pair-wise relatedness (R) within groups (females phase I, males phase I, etc.) was estimated in program Relatedness 5.0.8 (http://www.bioc.rice.edu/~kfg/Gsoft.html; Queller & Goodnight 1989). Pair-wise relatedness is a measure of the extent to which two individuals have alleles that are identical by descent relative to allele frequencies in the entire population and ranges from −1 to +1. A positive value signifies that two individuals share more alleles than expected by chance and hence are related, whereas values of zero or below signifies non-relatives (Queller & Goodnight 1989). For estimating the overall allele frequencies within NYE cougars, we used the largest number of samples available by including all residents (n=39) as well as 29 cougars of reproductive age killed by hunters within 100 km of the northern park boundary (table 1). All offspring were excluded to avoid over-representation of alleles (Queller & Goodnight 1989). Allele frequencies were then used to create a simulated set of 1000 pairs of unrelated individuals generated in program Kinship 1.3.1 (Goodnight et al. 1998) as a null distribution. Because pair-wise R-values are not independent, a one-tailed permutation test was employed to test whether distribution means were significantly different (Ratnayeke et al. 2002). To determine the distribution of R-values for first-order relatives we also conducted pair-wise comparisons for 53 mother–kitten pairs and 32 full-sibs (see electronic supplementary material).
3. Results
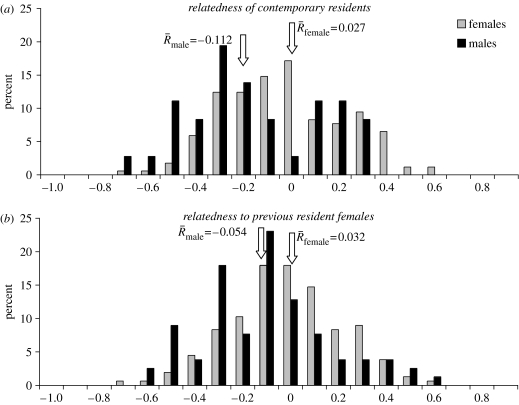
Consistent with expectations of male-biased dispersal, female cougars showed significantly higher R (R=0.03±0.02) than males (R=−0.11±0.05; p=0.002; figure 1a). R-values were thereby determined by comparing females and males within each study phase before combining data from both phases for each sex. We tested our first prediction that females and males differ by sharing more and fewer alleles than expected, respectively, by comparing their R-values to the null distribution of unrelated individuals. Contrary to our prediction, females did not share more alleles than expected (p=0.122), but the prediction of fewer alleles shared was met for males (p=0.004).
Figure 1.
Distribution of R values of male and female cougars in the northern Yellowstone ecosystem for (a) residents present during the same sampling period and (b) resident females 1988–1995 (phase I) and female and male residents 1998–2003 (phase II).
We tested our second prediction of sex-related differences in relatedness to previous female residents by calculating R-values of phase I females to female and to male residents present several years later (phase II). For females, relatedness was not greater than expected by chance (R=0.03±0.02; p=0.066), whereas males shared fewer alleles with phase I females than expected (R=−0.05±0.03, p=0.004; figure 1b). Thus, results again failed to support our prediction for females, but did support it for males.
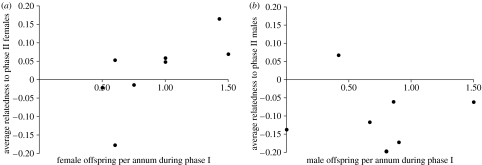
The genetic signal of female philopatry could be obscured if only a fraction of resident females are successful in leaving offspring that become residents themselves, which may apply to NYE cougars (Murphy 1998). We therefore predicted that female reproductive success would be positively correlated with genetic relatedness to subsequent generations of resident females, but we predicted that this would not be the case for resident males, because they are unlikely to be recruited from the NYE population. We determined the number of female and male offspring produced per year by eight phase I females that had been monitored for at least three reproductive seasons. Number of offspring was determined through field observation at the earliest possible time after birth, usually within a few months (Murphy 1998). If sex of the offspring was unknown (e.g. when kittens were known, but not captured) it was counted as 0.5 female and 0.5 male offspring to account for the entire litter size. Consistent with our prediction, we discovered a positive correlation between the number of female offspring produced annually by phase I females and their average relatedness to phase II females (figure 2a; r=0.819, p=0.013). Also as predicted, no such relationship between the number of male offspring produced and relatedness to phase II males was found (figure 2b; r=−0.006, p=0.887).
Figure 2.
Relationship between number of offspring produced by eight phase I females and their average relatedness to phase II residents. (a) Female offspring and phase II female residents (r=0.819, p=0.013); (b) male offspring and phase II male residents (r=−0.006, p=0.887).
4. Discussion
For male cougars, all results supported our predictions: male residents shared fewer alleles with each other (figure 1a) and with previous female residents (figure 1b), including those with high reproductive success (figure 2b). This is consistent with males generally immigrating from outside the NYE. Maximum dispersal distances of greater than 700 km have been documented in male cougars (Logan & Sweanor 2002) and we found that resident males often carried alleles that were uncommon in the NYE and that were not shared with other males. Thus, NYE males appear to originate in areas that are geographically distant enough to exhibit pronounced genetic differences. This lends support to the notion that inbreeding avoidance may represent the most important adaptive advantage of long-distance dispersal in male carnivores (Pusey 1987; Logan & Sweanor 2002).
Compared to males, NYE females were genetically more similar to each other, consistent with shorter dispersal distances. Still, our first two predictions were not supported for females: neither contemporary residents nor female residents separated by few generations shared on average more alleles than expected by chance, and thus must be considered unrelated (figure 1a,b). This means, that either philopatric behaviour is less pronounced in female cougars than formerly assumed or its genetic effects are outweighed by other factors. The fact that nine females but only one male that were born in the NYE also became residents there (T. K. Ruth & K. M. Murphy 2005, unpublished data), clearly underlines female fidelity to the natal area, as described from cougars elsewhere (Logan & Sweanor 2002). Furthermore, consistent with our third prediction, we were able to document the genetic legacy of females with high reproductive success (figure 2a), a relationship only expected under female philopatry. Thus, we have no reason to believe that female cougars on average disperse far beyond the natal area.
We suggest three factors that may explain a lack of relatedness among females. First, frequent introduction of new alleles by immigrating males may be sufficient in itself to prohibit elevated levels of allele sharing among females (Goudet et al. 2002). Second, although heritability of female reproductive success has been documented for other solitary feline species (Kelly 2001), this may not be the case in cougars. Regular local turnover of maternal lineages would tend to disrupt local clusters of related females. Third, although most dispersing females seem to stay within a few kilometres of their natal area (Logan & Sweanor 2002), such short distance dispersal of females may be effective in preventing spatial clustering of relatives. Further analyses are needed to distinguish among these possibilities, which may also work in combination.
Waser & Jones (1983) showed that philopatry, especially of female offspring, is widespread among solitary mammals and argued that its evolutionary significance had been underestimated. While young female cougars sometimes establish home ranges that overlap with those of their mothers or siblings (Logan & Sweanor 2002), our data indicate that the genetic effects of this phenomenon are rather inconsequential at the population level. Our results therefore suggest that dispersal distances in female cougars are generally large enough to reduce the chance of interaction among relatives. At the same time, we found that alleles of females with higher reproductive success became noticeably more abundant among subsequently resident females, indicating the potential for increased clustering of relatives. While cougar habitat in and around the NYE is more or less contiguous, this raises the question if the relatedness patterns we observed in females would change if habitat became fragmented. Owing to its shorter distance, dispersal in female cougars may be frustrated more easily if suitable habitat close to the natal area is not available (Logan & Sweanor 2002). Under such circumstances, gene flow would rely solely on male immigration and relatedness among local females would be likely to increase.
Acknowledgments
M. Hornocker and H. Quigley initiated the phase I and phase II cougar studies. Funding was provided by the J. Murdock Charitable Trust, National Fish and Wildlife Federation, Richard King Mellon Foundation, Charles Engelhard Foundation, Wildlife Conservation Society, Michael Cline Foundation, M. Comegys, Laura Moore Cunningham Foundation, Summerlee Foundation, Tim and Karen Hixon Foundation, National Geographic Society, L. Westbrook, Argosy Foundation, J. Hagenbuch, M. and A. Manship and The Bay Foundation. N.A. was supported by the UM BRIN summer undergraduate research program (NIH P20 RR-1645-02). We thank numerous field technicians for their dedicated effort, A. Saxton for kindly sharing statistical software, and two anonymous reviewers for comments.
Footnotes
Present address: National Park Service, Yellowstone National Park, WY 82190, USA.
Supplementary Material
Provides information regarding microsatellite amplification, descriptive statistics, and relatedness among first-order relatives
References
- Gompper M.E, Gittleman J.L, Wayne R.K. Dispersal, philopatry, and genetic relatedness in a social carnivore: comparing males and females. Mol. Ecol. 1998;7:157–163. doi: 10.1046/j.1365-294x.1998.00325.x. doi:10.1046/j.1365-294x.1998.00325.x [DOI] [PubMed] [Google Scholar]
- Goodnight, K. F., Queller, D. C. & Poznansky, T. 1998 Kinship 1.3.1. Available at http://www.bioc.rice.edu/~kfg/Gsoft.html
- Goudet J, Perrin N, Waser P. Tests for sex-biased dispersal using bi-parentally inherited genetic markers. Mol. Ecol. 2002;11:1103–1114. doi: 10.1046/j.1365-294x.2002.01496.x. doi:10.1046/j.1365-294X.2002.01496.x [DOI] [PubMed] [Google Scholar]
- Greenwood P.J. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 1980;28:1140–1162. [Google Scholar]
- Kelly M.J. Lineage loss in Serengeti cheetahs: consequences of high reproductive variance and heritability of fitness on effective population size. Conserv. Biol. 2001;15:137–147. doi:10.1046/j.1523-1739.2001.99033.x [Google Scholar]
- Logan K.A, Sweanor L.L. Island Press; Covelo, CA: 2002. Desert puma: evolutionary ecology and conservation of an enduring carnivore. [Google Scholar]
- Murphy, K. M. 1998 The ecology of the cougar (Puma concolor) in the northern Yellowstone ecosystem: interactions with prey, bears, and humans. Ph.D. thesis, University of Idaho.
- Packer C, Gilbert D.A, Pusey A.E, O'Brien S.J. A molecular genetic analysis of kinship and cooperation in African lions. Nature. 1991;351:562–565. doi:10.1038/351562a0 [Google Scholar]
- Perrin N, Goudet J. Inbreeding, kinship and the evolution of natal dispersal. In: Clobert J, Nichols J.D, Danchin E, Dondht A, editors. Dispersal. Oxford University Press; Oxford, UK: 2001. pp. 110–122. [Google Scholar]
- Pusey A.E. Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends Ecol. Evol. 1987;2:295–299. doi: 10.1016/0169-5347(87)90081-4. doi:10.1016/0169-5347(87)90081-4 [DOI] [PubMed] [Google Scholar]
- Queller D.C, Goodnight K.F. Estimating relatedness using genetic markers. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. [DOI] [PubMed] [Google Scholar]
- Ratnayeke S, Tuskan G.A, Pelton M.R. Genetic relatedness and female spatial organization in a solitary carnivore, the raccoon, Procyon lotor. Mol. Ecol. 2002;11:1115–1124. doi: 10.1046/j.1365-294x.2002.01505.x. doi:10.1046/j.1365-294X.2002.01505.x [DOI] [PubMed] [Google Scholar]
- Rogers L.L. Effects of food supply and kinship on social behavior, movements, and population growth of black bears in northeastern Minnesota. Wildl. Monogr. 1987;97:1–72. [Google Scholar]
- Van Horn R.C, Engh A.L, Scribner K.T, Funk S.M, Holekamp K.E. Behavioural structuring of relatedness in the spotted hyena (Crocuta crocuta) suggests direct fitness benefits of clan-level cooperation. Mol. Ecol. 2004;13:449–458. doi: 10.1046/j.1365-294x.2003.02071.x. doi:10.1046/j.1365-294X.2003.02071.x [DOI] [PubMed] [Google Scholar]
- Waser P.M, Jones W.T. Natal philopatry among solitary mammals. Q. Rev. Biol. 1983;58:355–390. doi:10.1086/413385 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Provides information regarding microsatellite amplification, descriptive statistics, and relatedness among first-order relatives