Abstract
Through non-genetic maternal effects, mothers can tailor offspring phenotype to the environment in which young will grow up. If juvenile and adult ecologies differ, the conditions mothers experienced as juveniles may better predict their offspring's environment than the adult environment of mothers. In this case maternal decisions about investment in offspring quality should already be determined during the juvenile phase of mothers. I tested this hypothesis by manipulating juvenile and adult maternal environments independently in a cichlid fish. Females raised in a poor environment produced larger young than females raised without food limitations, irrespective of the feeding conditions experienced during adulthood. This maternal boost was due to a higher investment in eggs and to faster larval growth. Apparently, mothers prepare their offspring for similar environmental conditions to those they encountered as juveniles. This explanation is supported by the distribution of these fishes under natural conditions. Juveniles live in a different and much narrower range of habitats than adults. Therefore, the habitat mothers experienced as juveniles will allow them to predict their offspring's environment better than the conditions in the adult home range.
Keywords: maternal effects, ontogeny, egg size, cichlids, life history
1. Introduction
Non-genetic maternal effects are widespread and strongly influence the fitness of mothers and offspring (reviewed in Mousseau & Fox 1998; Lindström 1999; Lummaa & Clutton-Brock 2002). Egg size (Bernardo 1996; Mousseau & Fox 1998) and quality (e.g. Schwabl et al. 1997; Blount et al. 2002) are important maternal effects, which can determine the entire life histories of offspring (Lindström 1999). Maternal effects may depend on maternal condition (e.g. Blount et al. 2002) and on the conditions in the current environment (Mousseau & Fox 1998; Lindström 1999) or during preceding breeding events (Reznick & Yang 1993).
While this appears to be adequate in some cases, in many animals successive life stages use different habitats (reviewed in Werner & Gilliam 1984), or they use resources differently within the same habitat (e.g. Lind & Welsh 1994). If juvenile and adult ecologies are uncorrelated, cues from the current conditions are a poor predictor of the environment offspring are likely to encounter (Bernardo 1996). In such cases, the conditions a female encountered as a juvenile may allow her to predict her offspring's environment with a much higher precision. Consequently, maternal investment decisions should be determined by a mother's early development (sensu Lindström 1999), but should be independent of the environmental conditions in which young are produced.
I tested this hypothesis experimentally with the African cichlid Simochromis pleurospilus by independently varying the resource availability of juveniles and adult females. In the field, I tested the prediction that the habitat use of adult females and juveniles differs, so that the environmental conditions experienced these stages are rather independent of each other.
2. Material and methods
I raised 120 fishes (among them 55 females) of the maternally mouthbrooding cichlid S. pleurospilus, each in a separate tank, exposing aliquot numbers to high-food (H) and low-food (L) conditions (juvenile treatment, J) (see electronic supplementary material A and Taborsky (2006) for details on experimental set-up). Fishes were fed 6 days a week with standardized agarose gel cubes containing an amount of Tetramin flake food corresponding to 12 or 4% of mean body weight plus 5% Spirulina algae. I adjusted the food amounts to increasing body weight every 14 days. The juvenile treatment took on average 265 days and covered the period between independence of young (mean age 29 days) and the end of the first breeding attempt. Afterwards, half of all reproductively active females (n=23) were switched to the opposite treatment, while the remaining 23 females stayed on the original ration (adult treatment, A). Siblings were assigned alternately to high- or low-food ration as juveniles, and to switched or original ration during the adult treatment to achieve equal brood splitting. The resulting treatment groups were denoted as HH (n=13), HL (n=10), LH (n=11) and LL (n=12). For each successive brood a female was mated with a different male (Taborsky 2006 gives details about schedules of male presence in female tanks). About 50% of females never raised a brood successfully despite spawning several clutches. Females remained in the adult treatment until they stopped producing further clutches (mean reproductive lifespan in experiment: 264.5 days; Taborsky 2006).
Brood care of S. pleurospilus consists of two phases, two weeks of continuous incubation when young use up yolk reserves (‘first incubation phase’) and two weeks when young are periodically released from the mouth for feeding (’second incubation phase’). Total length (TL; nearest 0.5 mm) and weight (W; nearest 0.01 g) of females and young (TL: nearest 0.1 mm, W: nearest 0.0001 g) were measured at the end of both incubation phases. I calculated Fulton's condition factor K as K=100×W/TL3, and specific growth rates of young (SGR) as ln(TL2/TL1)/t, where TL1 and TL2 are lengths at the beginning and end of the second incubation phase, respectively, and t is the duration of this phase. As SGR depends on absolute size, it was corrected for TL1. The experiment lasted from November 2001 to May 2004. One clutch each was collected from all females that spawned during the last six months of this period (3HH, 4HL, 4LH and 4LL females) to measure egg mass. Clutches could not be collected earlier, as this would have interfered with the measurement of reproductive rates of females (see Taborsky 2006). Mean clutch mass was calculated from individual egg dry mass measured to the nearest 0.0001 g.
I calculated two-way analyses of variance with juvenile treatment (J) and adult treatment (A) as independent factors and individual females as independent units of analysis (using female means of clutch means). Female size and body condition was not related to size or weight of young after the first and second incubation phase, and was therefore not included as a covariate in the ANOVAs.
I determined the size-frequency distribution of juveniles and females across the natural depth range of S. pleurospilus by transect counts along a 150 m stretch of pebble shore in Mbete Bay near Mpulungu, Zambia (see electronic supplementary material A for details on survey methods). The mean distributions of four study years are presented here. In the lab experiment, females started to reproduce at a mean size of 5.7 cm TL (Taborsky 2006). Therefore, I considered individuals in size classes up to 4.5–5.4 cm as ‘juveniles’ and individuals above 5.5 cm as ‘adults’.
3. Results
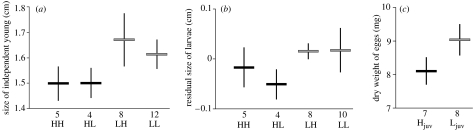
When mothers had been raised with little food, offspring were on average 1.4 mm larger at the end of brood care than when mothers had been well fed, irrespective of the ration mothers received during adulthood (figure 1a; table 1). The larger size of young was not explained by extended brood care, as the results did not change when controlling for the age of young (table 1). Rather young of poorly reared mothers grew faster during the second incubation phase (independent of their size at the beginning of this phase; table 1). The condition factor K of young was not affected by treatment (table 1), showing that faster growth was not compromised by a slower increase in body mass.
Figure 1.
Total length of offspring at the end of (a) second incubation phase, (b) first incubation phase (residuals of the model with offspring age as covariate are shown) and (c) egg dry mass of females (mean±s.e. of brood means).
Table 1.
Analyses of variance testing for the effects of juvenile (J) and adult treatment (A) and their interaction on dependent variables associated with offspring phenotype of experimental females.
| TL after second incubation phase (cm) | TL after second phase corr. for age (cm) | K after brood care (g cm−3) | TL after first incubation phase corr. for age | residual SGRa during second incubation phase (% d−1) | ||
|---|---|---|---|---|---|---|
| d.f. | 3, 25 | 4, 24 | 3, 25 | 4, 22 | 3, 23 | |
| R2 | 0.30 | 0.53 | 0.008 | 0.78 | 0.39 | |
| full model | F | 3.64 | 6.80 | 1.07 | 19.42 | 4.91 |
| p | 0.026 | 0.001 | 0.379 | <0.001 | 0.009 | |
| covariate offspring age | F | 11.63 | 64.0 | |||
| p | 0.002 | <0.001 | ||||
| main effects | ||||||
| J | F | 10.28 | 16.20 | 0.13 | 5.93 | 9.15 |
| p | 0.004 | <0.001 | 0.721 | 0.023 | 0.006 | |
| A | F | 0.35 | 0.42 | 2.81 | 0.84 | <0.001 |
| p | 0.559 | 0.552 | 0.106 | 0.370 | 0.98 | |
| J×A | F | 0.40 | 1.25 | 0.001 | 0.88 | 4.83 |
| p | 0.530 | 0.274 | 0.975 | 0.359 | 0.038 |
Female means of residuals of the regression between SGR and initial TL of young (clutch means) at the beginning of the second incubation phase.
Young of poorly raised mothers were already larger for their age after the first incubation phase (figure 1b; table 1), when larvae depend entirely on yolk reserves. Accordingly, females raised with little food laid eggs with a higher dry mass (ANOVA, F1,13=8.74, p=0.011; figure 1c). Egg mass was not related to female length (regression analysis: R2=0.02, p=0.64, d.f.=1,13) or body condition (R2=0.04, p=0.52, d.f.=1,12).
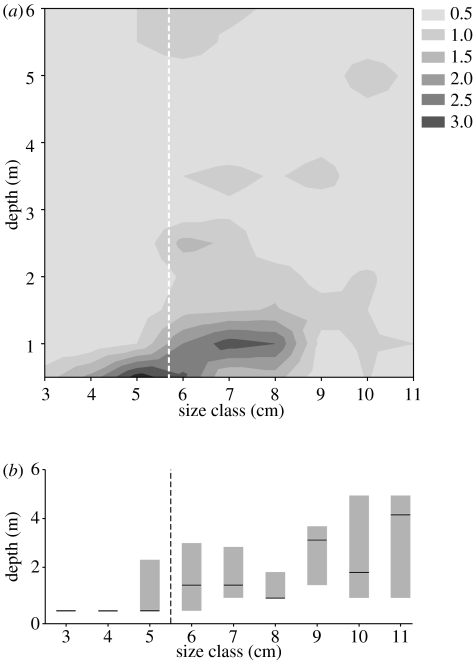
In their natural habitat, the majority of juveniles (less than 5.5 cm) stayed at a depth of 0.5 m and were rarely found below 1 m (median depth: 0.5 m, inter-quartile range (i.q.r.) 0.5–1; figure 2). In contrast, fishes of adult size occurred predominantly in deeper water (median: 1.5 m, i.q.r. 1.0–3.5; figure 2). Juvenile and adult habitat use differed with respect to depth distribution (chi-square test, χ52=36.94, p<0.001; frequencies at 3 m and below were pooled because of low expected frequencies in these depths) and central tendency of depth distribution (median test, χ12=11.16, p<0.001).
Figure 2.
(a) Size-depth distribution of S. pleurospilus (mean of four study seasons); grey shades indicate frequencies of fishes recorded for each size class and depth. (b) Medians and quartiles for the depth distributions of 1 cm size classes. Stippled lines indicate size at maturity.
Above 0.5 m water depth, only small specimens (1.5–4.4 cm) were found. As they could not be counted by transect swimming, these numbers are not included in the results (separate dataset of shallow-water counts given in electronic supplementary material A).
4. Discussion
At the end of brood care, young of S. pleurospilus mothers raised on a poor diet were on average 8.8% longer than young of mothers raised without food limitation. This size difference is likely to affect offspring fitness, as even a size advantage of 3.3% can significantly increase the survival chances of fish larvae (McCormick & Hoey 2004). This is the first experimental evidence showing that maternal effects induced by pre-reproductive conditions can be similarly expressed in markedly different reproductive environments, while not being influenced by the conditions in which females produce their young. Moreover, the reported size differences of young were based on up to four successful broods spread over female reproductive lifespan, suggesting that such maternal effects can persist during the entire adulthood even in iteroparous, long-lived animals.
Poorly raised females paid costs for producing larger young by having smaller clutches, while there was no indication of such a trade-off in females raised on the high-food diet (Taborsky 2006). If females raised on a poor diet had resulted in poorer quality adults than females raised on a rich diet, the former may have been more affected by the costs of reproduction than the latter.
S. pleurospilus females raised with little food apparently used two different mechanisms to boost the size of young. (i) They produced eggs with a higher energy content as indicated by higher egg dry mass. (ii) Young grew faster during the second incubation phase, when most yolk is used up and they already use external food sources. The mechanism responsible for faster growth needs still to be clarified.
In several fishes, insects and amphibians larger offspring have survival advantages under adverse growth conditions, while under benign conditions smaller young do equally well (Hutchings 1991; Mousseau & Fox 1998; Einum & Fleming 1999) or better (Kaplan 1992; Rotem et al. 2003). Therefore, the experimental results strongly suggest that S. pleurospilus females prepared their young for similar environmental conditions as they had encountered themselves as juveniles. In humans, it has been hypothesized that predicting the quality of the offspring environment incorrectly may have detrimental consequences for offspring health and survival (Bateson et al. 2004). The conditions mothers experienced as juveniles may predict their offspring's environment better than current environmental cues, if (i) environmental conditions for adults and juveniles vary independently and (ii) juvenile conditions of successive generations are similar. When juveniles and adults have entirely different ecologies like in metamorphosing animals or anadromous fishes, some species appear indeed to adjust offspring phenotype to their juvenile environment (Jonsson et al. 1996; Rotem et al. 2003) while others do not (Fox et al. 1995).
In contrast, juvenile and adult S. pleurospilus co-occur along the rocky shores of Lake Tanganyika and use the same major food source, filamentous turf algae. However, juveniles mainly live in shallow water using only a narrow range of water depths, while after maturity females mainly use deeper habitats for breeding (figure 2a). The juvenile habitat has a high productivity of turf algae, while productivity varies by at least two orders of magnitude over the depth range inhabited by adults (Taborsky 1999). Owing to this large variation the ability to predict food availability for offspring reliably is limited if females use cues from their ambient environment. A much better estimate can be achieved if females use the growth conditions experienced during their own juvenile phase.
As differential habitat use of juveniles and adults is very widespread in animals, parents will often not be able to predict the conditions for the early life stages of offspring by using cues from their current environment. Therefore, I predict that it is a common parental strategy to base decisions about the investment in individual offspring more strongly on their own early environment than on present conditions. When studying the origin and function of parental effects, it is hence important that the influence on parents of the environment experienced during early development is incorporated.
Acknowledgments
I am indebted to D. Heg, I. Hamilton and M. Taborsky for helpful comments on the manuscript, to S. Aschwanden, Z. Bachar, D. Bonfils, R. Eggler C. Grüter, N. Hirt, S. Immler, S. Lehner, M. Massironi, G. Pachler, T. Reichlin, R. Schürch and P. Vonlanthen for assistance with data collection and analysis and to the Austrian Science Fund (FWF grant P14327-B06) and the Forschungsstiftung, University of Bern (48/2003) for financial support.
Supplementary Material
References
- Bateson P, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. doi:10.1038/nature02725 [DOI] [PubMed] [Google Scholar]
- Bernardo J. Maternal effects in animal ecology. Am. Zool. 1996;36:83–105. [Google Scholar]
- Blount J.D, Surai P.F, Nager R.G, Houston D.C, Moller A.P, Trewby M.L, Kennedy M.W. Carotenoids and egg quality in the lesser black-backed gull Larus fuscus: a supplemental feeding study of maternal effects. Proc. R. Soc. B. 2002;269:29–36. doi: 10.1098/rspb.2001.1840. doi:10.1098/rspb.2001.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einum S, Fleming I.A. Maternal effects of egg size in brown trout (Salmo trutta): norms of reaction to environmental quality. Proc. R. Soc. B. 1999;266:2095–2100. doi:10.1098/rspb.1999.0893 [Google Scholar]
- Fox C.W, Waddell K.J, Mousseau T.A. Parental host plant affects offspring life histories in a seed beetle. Ecology. 1995;76:402–411. [Google Scholar]
- Hutchings J.A. Fitness consequences of variation in egg size and food abundance in brook trout Salvelinus fontinalis. Evolution. 1991;45:1162–1168. doi: 10.1111/j.1558-5646.1991.tb04382.x. [DOI] [PubMed] [Google Scholar]
- Jonsson N, Jonsson B, Fleming I.A. Does early growth cause a phenotypically plastic response in egg production of Atlantic salmon? Funct. Ecol. 1996;10:89–96. [Google Scholar]
- Kaplan R.H. Greater maternal investment can decrease offspring survival in the frog Bombina orientalis. Ecology. 1992;73:280–288. [Google Scholar]
- Lind A.J, Welsh H.H. Ontogenetic changes in foraging behaviour and habitat use by the Oregon garter snake Thamnophis atratus hydrophilus. Anim. Behav. 1994;48:1261–1273. doi:10.1006/anbe.1994.1362 [Google Scholar]
- Lindström J. Early development and fitness in birds and mammals. Trends Ecol. Evol. 1999;14:343–348. doi: 10.1016/s0169-5347(99)01639-0. [DOI] [PubMed] [Google Scholar]
- Lummaa V, Clutton-Brock T. Early development, survival and reproduction in humans. Trends Ecol. Evol. 2002;17:141–147. doi:10.1016/S0169-5347(01)02414-4 [Google Scholar]
- McCormick M.I, Hoey A.S. Larval growth history determines juvenile growth and survival in a tropical marine fish. Oikos. 2004;106:225–242. doi:10.1111/j.0030-1299.2004.13131.x [Google Scholar]
- Mousseau T.A, Fox C.W. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. doi:10.1016/S0169-5347(98)01472-4 [DOI] [PubMed] [Google Scholar]
- Reznick D, Yang A.P. The influence of fluctuating resources on life-history patterns of allocation and plasticity in female guppies. Ecology. 1993;74:2011–2019. [Google Scholar]
- Rotem K, Agrawal A.A, Kott L. Parental effects in Pieris rapae in response to variation in food quality: adaptive plasticity across generations? Ecol. Entomol. 2003;28:211–218. doi:10.1046/j.1365-2311.2003.00507.x [Google Scholar]
- Schwabl H, Mock D.W, Gieg J.A. A hormonal mechanism for parental favouritism. Nature. 1997;386:231. doi:10.1038/386231a0 [Google Scholar]
- Taborsky B. Size-dependent distribution in littoral fish: optimization or competitive exclusion? In: Almada V.C, Oliveira R.F, Goncalves E.J, editors. Behaviour and conservation of littoral fishes. ISPA; Lisboa: 1999. pp. 351–376. [Google Scholar]
- Taborsky B. The influence of juvenile and adult environments on life history trajectories. Proc. R. Soc. B. 2006;273 doi: 10.1098/rspb.2005.3347. doi:10.1098/rspb.2005.3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E.E, Gilliam J.F. The ontogenetic niche and species interactions in size structured populations. Annu. Rev. Ecol. Syst. 1984;15:393–425. doi:10.1146/annurev.es.15.110184.002141 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.