Abstract
To analyze the mechanisms of entry of human herpesvirus 8 (HHV-8), we established a reporter cell line T1H6 that contains the lacZ gene under the control of the polyadenylated nuclear RNA promoter, known to be strongly activated by a viral transactivator, Rta. We found that infection with cell-free virus, as well as cocultivation with HHV-8-positive primary effusion lymphoma cell lines, activated the lacZ gene of T1H6 in a sensitive and dose-dependent manner. Addition of Polybrene and centrifugation enhanced, but polysulfonate compounds inhibited, the HHV-8 infectivity. RGD-motif-containing polypeptides and integrins did not decrease the infectivity, suggesting the presence of an additional cellular receptor other than the reported one. The entry was dependent on pH acidification but not on the clathrin pathway. Although conditioned media obtained from human immunodeficiency virus (HIV)-infected cells did not have any effect on the early steps of HHV-8 infection, intracellular expression of a proviral HIV type 1, but not of Tat alone, increased the HHV-8-dependent reporter activation slightly, suggesting a potential of HIV-mediated enhancement of an early step of HHV-8 infection.
Attachment and entry represent the first essential steps of viral replication. Enveloped viruses have evolved two main pathways to mediate their entry into the cells after attachment to cell surface moieties (reviewed in reference 25). The first one, low-pH-dependent pathway, involves endocytosis of viral particles followed by viral-cell membrane fusion in endosomes or lysosomes. This fusion is triggered by an acidic-pH-dependent conformational change of viral glycoprotein(s) and allows release of capsid into the cytoplasm. Entry of vesicular stomatitis virus (VSV), the prototype rhabdovirus, exemplifies this pathway. In contrast, in the second one, pH-independent pathway, viral-cell membrane fusion takes place on the plasma membrane at neutral pH. Most retroviruses and paramyxoviruses use this pathway. The pH-independent entry was also demonstrated for herpesviruses, herpes simplex virus (55), and cytomegalovirus (CMV) (16). However, Epstein-Barr virus (EBV) uses membrane fusion both in endosomes and on the plasma membrane differentially (40).
Human herpesvirus 8 (HHV-8), also known as Kaposi's sarcoma-associated herpesvirus (KSHV), is a member of the gammaherpesvirus subfamily and is etiologically associated with Kaposi's sarcoma, primary effusion lymphoma (PEL), and multicentric Castleman's disease (reviewed in references 1 and 11). It interacts with target cells by binding of glycoprotein B (gB) and K8.1 with glycosaminoglycans, such as heparan sulfate, on the cell surface (5, 10, 54). Recent studies found that gB also bound to α3β1 integrin through its RGD motif and induced ERK signaling pathway, implicating that α3β1 integrin functions as a cellular receptor for HHV-8 entry (6, 42). One of the major constraints to study the entry mechanisms of HHV-8 is a lack of fully permissive cell lines to conduct traditional virological assays by measuring virus titers, although a few cell lines, including 293 cells, some carcinoma and human papillomavirus-transformed cell lines, and immortalized endothelial cells, support permissive infection at a low level (32, 41, 45). The currently available assays that allow titration of HHV-8 include (i) enumeration of cells expressing immediate-early antigen ORF73 after infection, (ii) a plaque assay using primary dermal microvascular endothelial cells (15), (iii) quantitative PCR assays to measure encapsidated viral DNA (43, 50), and (iv) an enzyme-linked immunosorbent assay (ELISA) based on virion capture (30). However, some of these assays are laborious or time consuming. In addition, it is not guaranteed that the genome and particle numbers measured by PCR and the virion-capture ELISA reflect infectious particle numbers.
Establishment of the HHV-8 reporter cell line.
In this study, we developed a new assay for HHV-8 titration by establishing a reporter cell line and characterized the factors that affect HHV-8 infectivity. For this purpose, we used Rta-dependent activation of the polyadenylated nuclear (PAN) RNA promoter. Rta encoded by open reading frame (ORF) 50 is a key HHV-8 regulator of the switch from a latent to lytic program, and it induces the expression of a number of HHV-8 and cellular promoters and is sufficient to trigger the entire lytic infection process (17, 33, 47, 51). PAN RNA is the most abundant transcript whose expression is activated by Rta through the responsive element RRE (49). We constructed a reporter plasmid, pβgal-T1.1, in which the PAN promoter region −122 to +14 was cloned between XhoI and HindIII sites of pβgal-basic (BD Bioscience Clontech, Palo Alto, Calif.). 293T cells were transfected with pβgal-T1.1 and a plasmid encoding hygromycin B phosphotransferase at a 50:1 ratio. The clone T1H6 was selected from 46 hygromycin B-resistant clones based on β-galactosidase activities induced by transient transfection of each clone with pCMV-ORF50 expressing Rta (47). β-Galactosidase activities were obtained by a chemiluminescent assay reaction (Luminescent β-galactosidase Detection Kit II, BD Bioscience Clontech) followed by measurement of relative light units with a luminometer (TD-20/20; Turner Designs, Sunnyvale, Calif.) using a sensitivity setting that allows a linear reading over a 3-log range. Transfection of pCMV-ORF50 activated the reporter gene of T1H6 cells in a dose-dependent manner (Fig. 1A). Treatment with 20 ng of 12-O-tetradecanoylphorborl-13-acetate (TPA)/ml did not activate the PAN promoter in 293T cells transiently transfected with pβgal-T1.1 (data not shown) or in T1H6 cells (Fig. 1A). The stability of T1H6 cells for the Rta-dependent activation was confirmed by another transient transfection assay after culturing for more than 1 month.
FIG. 1.
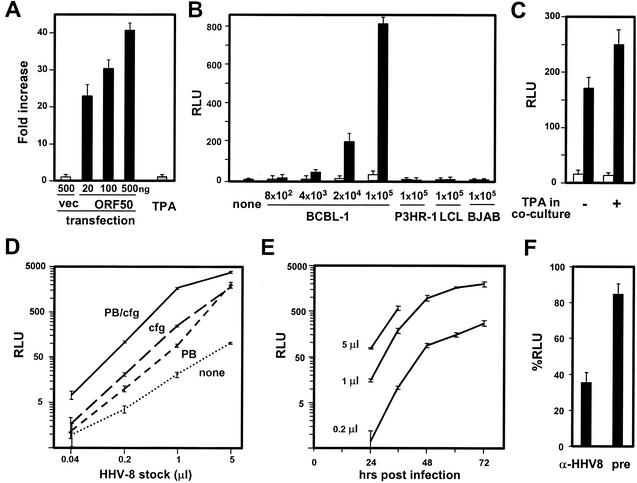
(A) Rta-dependent activation of T1H6 reporter cells. T1H6 cells (1 × 105 cells per well) were plated in a 24-well plate, and 1 day later, they were transfected with the indicated amount of pCMV-Script (vec) or pCMV-ORF50 (ORF50) by the calcium-phosphate method or treated with 20 ng of TPA/ml. β-Galactosidase activities of the cells were measured by a chemiluminescent assay 2 days after transfection or addition of TPA. β-Galactosidase activities of transfected cells were compared with that of untreated cells and are shown as means and standard deviations of fold increases from the triplicate experiments. (B) Specificity and detection limit in cocultivation. T1H6 cells (4 × 104 cells/well) were plated in 48-well plates, and 1 day later, they were overlaid with the indicated numbers of untreated (open bars) and TPA-treated (closed bars) cells and then cultured for an additional 3 days in the absence of TPA. Means and standard deviations of β-galactosidase activities of the cells from the triplicate experiments are shown as relative light units (RLU). P3HR-1 (ATCC HTB-62), EBV-positive Burkett's lymphoma cell line; LCL (LCL721; ATCC CRL-1855), EBV-transformed lymphoblastoid cell line; BJAB (31), EBV-negative Burkett's lymphoma cell line. (C) Little effect of TPA during cocultivation. BCBL-1 cells cultured in the absence (open bars) or presence (closed bars) of TPA for 3 days were washed with TPA-free medium, overlaid on T1H6 cells, and then cultured in the absence or presence of TPA for 2 days. Means and standard deviations of β-galactosidase activities of the cells from the triplicate experiments are shown. (D) Centrifugal enhancement of free-virus infection and detection limit of cell-free virus. Partially purified virus stock was prepared by the following procedures. (i) BCBL-1 cells were cultured in the presence of TPA for 7 days; (ii) culture supernatant was prepared by removing cells by centrifugation; (iii) the supernatant was passed through a 0.45-μm-pore-size membrane; (iv) virus particles were pelleted by centrifugation (15,000 × g, 16 h); and (v) the virus particles were resuspended in 1/200 of the original volume. Therefore, 1 μl of the HHV-8 stock was derived from 200 μl of the original culture supernatant. The number of infectious units of the stock used here was ∼2 × 104 per microliter. T1H6 cells in 48-well plates (8 × 104 cells/well) were infected with the indicated volume of the virus stock in the absence or presence of 8 μg of Polybrene (PB)/ml, with or without centrifugation at 400 × g for 30 min (cfg). After incubation at 37°C for 90 min, the inoculums were removed and washed with medium, and cells were then cultured for 3 days. Means and standard deviations of β-galactosidase activities of the infected cells from the triplicate experiments are shown. (E) Time course of activation. T1H6 cells were infected with 5, 1, and 0. 2 μl of the same stock in the presence of PB and centrifugal enhancement, as described above. Infected cells were harvested at the indicated time points, and means and standard deviations of β-galactosidase activities from triplicate experiments are shown. (F) Neutralization of infection with anti-HHV-8 serum. Preimmune rabbit serum (pre) and rabbit anti-HHV-8 virion serum (α-HHV8) were incubated at 56°C for 30 min. HHV-8 (0. 2 μl) was incubated with either medium or these sera (a 1:10 dilution in 20 μl of final volume) for 30 min at 37°C and then used for infection of T1H6 cells. Three days after infection, β-galactosidase activities were measured and are shown as %RLU by using the activities obtained from infection with HHV-8 incubated in the absence of serum as a 100% control.
Specificity and sensitivity of the reporter cell line.
PEL cell lines, including BCBL-1 (46), JSC-1 (12), and BC-1 (13), and other EBV-positive and -negative B-cell lines were cultured in the presence or absence of TPA for 3 days, washed with TPA-free medium, and then cocultured with T1H6 cells for 3 days. Cocultivation with TPA-treated PEL cell lines activated the lacZ gene of T1H6 cells (Fig. 1B and data not shown). On the other hand, untreated PEL cell lines and HHV-8-negative cells did not activate the reporter gene (Fig. 1B), demonstrating the specificity of this assay. The detection limit for the lytically infected BCBL-1 cells was ∼100 cells, because ∼1,000 cells of TPA-induced BCBL-1 cells (Fig. 1B) with ∼10% of lytic infection (the percentage was based on immunofluorescence assay with anti-K8.1 antibody) was enough to provide the measurable signal. The signal was detectable after cocultivation for 2 days. Addition of TPA during cocultivation of untreated BCBL-1 cells with T1H6 cells for 2 days did not enhance the reporter gene activation (Fig. 1C), indicating that the Rta-dependent late phase of lytic infection, probably virion production, is required to activate the reporter gene. Next, the reporter gene activation was shown by infection of T1H6 cells with cell-free virus stocks prepared from culture supernatant of TPA-treated BCBL-1 cells. Polybrene during attachment increased the efficiency of cell-free virus infection (Fig. 1D), which is consistent with an earlier study (32). Additively, a low-speed centrifugation during attachment enhanced HHV-8 infectivity (Fig. 1D), similar to that with CMV (27). The detection limit of cell-free virus was ∼1,000 infectious units (IU), because the virus stock used here had ∼2 × 104 IU/μl, as determined by counting ORF73-positive cells in an immunofluorescent assay after infection of 293T cells, as described previously (28). β-Galactosidase activities were saturated with a multiplicity of infection of more than 1 (5 μl of the stock for 8 × 104 cells per well). IU measured on endothelial cells also correlated well with β-galactosidase activities in the reporter cell assay (L. Krug, N. Inoue, and M. K. Offermann, unpublished data). β-Galactosidase activities were detectable at 24 h postinfection (p.i.) and gradually reached a plateau by 72 h p.i. (Fig. 1E). Infection at a higher multiplicity of infection decreased cell viability after 24 h p.i. Treatment of cell-free virus with rabbit antiserum against purified HHV-8 virions, but not with preimmune serum, gifts from K. G. Kousoulas (Louisiana State University), decreased β-galactosidase activities significantly, confirming that the assay is HHV-8 specific (Fig. 1F).
Polysulfonate compounds, but not RGD-containing molecules, inhibit HHV-8 infectivity.
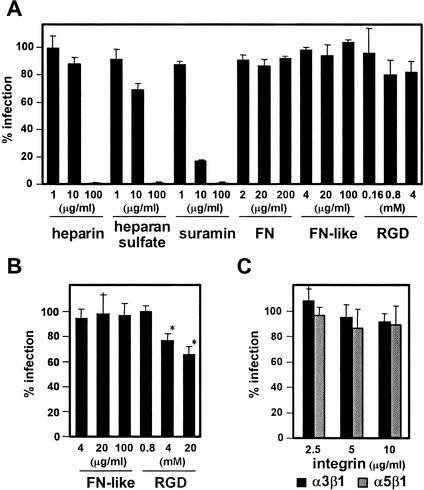
By using the reporter cell line, we first examined the initial interaction of HHV-8 with cell surface moieties. Heparan sulfate inhibited HHV-8 infectivity in T1H6 cells (Fig. 2A), confirming the previous studies (5, 10). Suramin, a symmetrical polysulfate naphthylamine derivative of urea, has anti-herpes simplex virus and anti-CMV properties (3) and inhibited HHV-8 infectivity also (Fig. 2A). Thus, the reporter cell assay is useful for screening of HHV-8 antiviral drugs. Next, we examined the inhibitory effects of RGD peptide, fibronectin (FN)-like polymer that contains 13 RGD motifs (21), and FN (Sigma, St. Louis, Mo.) on HHV-8, because RGD peptides and FN inhibited HHV-8 infectivity on human foreskin fibroblast cells (6). However, a detectable decrease of infectivity was not observed with FN and FN-like polymer under two different conditions, namely, a 2-h incubation in the presence of these molecules during infection (Fig. 2A) and a 2-h incubation prior to infection (Fig. 2B and data not shown). The highest concentration of FN used, 200 μg/ml, was more than fourfold the concentration used in the published report (6). Although RGD peptide decreased infectivity slightly, strong cell toxicity was observed after treatment with ≥4 μg of the peptide/ml. T1H6 cells transduced with a retrovirus vector expressing luciferase, LLRN (BD Bioscience Clontech), were treated with RGD peptide in the absence of HHV-8 infection. The decrease of luciferase activities after the treatment was comparable with that of β-galactosidase activities after HHV-8 infection, confirming that cell toxicity rather than inhibition of HHV-8 infectivity decreased the β-galactosidase activities (data not shown). We also examined whether soluble α3β1 or α5β1 integrins (Chemicon International, Temecula, Calif.) inhibit HHV-8 infectivity. Although it was previously reported that 5 μg of soluble α3β1, but not α5β1, integrins/ml inhibited HHV-8 infectivity on human foreskin fibroblast cells by >70% (6), there was no significant inhibition on T1H6 cells (Fig. 2C). Therefore, it is possible that T1H6 cells derived from 293T cells express an additional cellular receptor for HHV-8 infection other than α3β1 integrin.
FIG. 2.
Inhibition of HHV-8 infectivity by polysulfonate compounds but not RGD-motif-containing molecules. T1H6 cells (4 × 104 cells/well) were plated in 48-well plates, one day later infected with 1 μl of the HHV-8 stock in the presence of the indicated amount of inhibitors (FN, fibronectin; FN-like, FN-like polymer containing 13 RGD motifs; RGD, RGD peptide). (A) T1H6 cells were incubated in the presence of inhibitors with HHV-8 inoculums for 2 h. (B and C) The cells were also incubated in the presence of the indicated amount of FN-like polymer and RGD peptide at 4°C (B) or in the presence of soluble α3β1 and α5β1 integrins at 37°C (C) for 90 min, washed with the inhibitor-free medium, and then incubated with the HHV-8 inoculums for 2 h (B, C). After removal of inoculums, the cells were incubated for 52 h (A to C). Means and standard deviations from triplicate experiments are shown. The standard curve was obtained by infection of serial twofold dilutions of the virus stock, and RLU units were converted into % infection by using infection of 1 μl of the virus stock in the absence of inhibitors as a 100% control. Strong cell toxicity was observed after treatment with ≥4 μg of the peptide/ml (*).
HHV-8 entry is pH dependent.
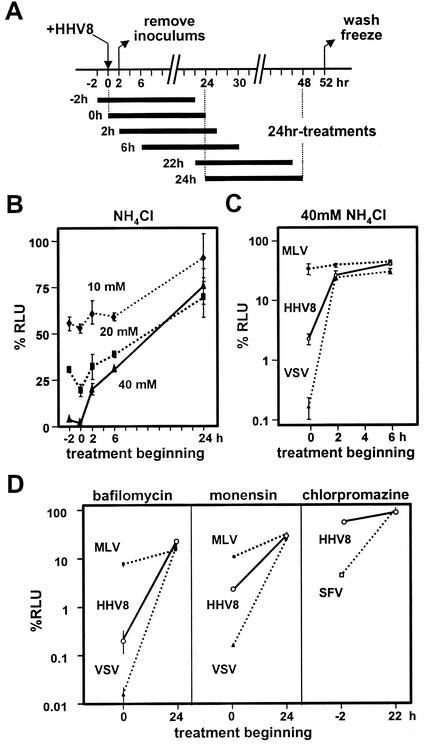
To examine whether HHV-8 entry is dependent on pH acidification, T1H6 cells were treated with 10 to 40 mM ammonium chloride for 24 h beginning at various time points (Fig. 3A). The treatment at the early stage of infection (<2 h p.i.) significantly decreased β-galactosidase activities (Fig. 3B). To ensure that the conditions used were optimal for this type of cell, retrovirus vectors pseudotyped with VSV-G and with amphotropic murine leukemia virus (MLV) envelope were used as positive and negative controls, because it is well known that VSV, but not amphotropic MLV, requires endocytosis and pH acidification for their entry (25), and retrovirus vectors pseudotyped with envelope glycoprotein(s) from heterologous virus display the characteristics of entry of the heterologous viruses (4, 14, 36, 48). The VSV- and MLV-pseudotyped retrovirus vectors were prepared by transient transfection of GP-293luc (packaging cell line for production of LLRN retrovirus vector; BD Bioscience Clontech) with pVSV-G (BD Bioscience Clontech) and pPAM3 (39), respectively, collection of culture supernatants 2 days later, and passage through 0.45-μm-pore-size filters. Infectivity of the retrovirus vectors on T1H6 cells was measured by transduction of luciferase, as described previously (29). The identical treatment with ammonium chloride inhibited infectivity of HHV-8 and of VSV-pseudotyped, but not of MLV-pseudotyped, retrovirus at the early, but not the late, stage of infection (Fig. 3C), demonstrating that HHV-8 entry is pH dependent. The pH dependence is also confirmed by inhibition with bafilomycin A1 (an inhibitor of vascular H+ATPase) and monensin (an ionophore that blocks endosomal acidification) (Fig. 3D). Next, we examined whether this pH-dependent infection uses the clathrin-dependent pathway. It is widely accepted that Semliki Forest virus (SFV) requires this pathway (18, 19). Chlorpromazine, an inhibitor of the clathrin-dependent endocytosis, decreased infectivity of a recombinant SFV expressing β-galactosidase that was prepared as described previously (28) in 293T cells, the parental cells of T1H6. Under the same condition, chlorpromazine did not inhibit HHV-8 infection (Fig. 3D, right). HHV-8 infection was not affected by a 24-h treatment of T1H6 cells with nystatin (25 μg/ml), an inhibitor of caveolae-dependent endocytosis (data not shown). The conditions used were reported to inhibit filovirus infection in 293T cells effectively (20). Thus, it is likely that the HHV-8 entry requires neither clathrin- nor caveolae-dependent endocytosis pathway. Further studies on HHV-8 infection of endothelial cells and B cells are required to warrant that our results can be generalized, because previous studies cautioned cell-type-specific usage of entry pathways (35, 40).
FIG. 3.
HHV-8 entry is pH dependent but not clathrin dependent. (A) Procedures to evaluate effects of a 24-h incubation of T1H6 cells in the presence of inhibitors. Beginning time points of 24-h incubation treatments are indicated relative to the time point when HHV-8 was added (0 h). (B) T1H6 cells were infected with HHV-8 and cultured in the presence of 10, 20, and 40 mM ammonium chloride for 24 h; their β-galactosidase activities are shown as %RLU by using the activities obtained from untreated cells as a 100% control. Means and standard deviations from triplicate experiments are shown. (C) T1H6 cells were infected with HHV-8, with LLRN retrovirus vectors pseudotyped with VSV-G (VSV), and with amphotropic MLV envelope (MLV) and cultured in the presence of 40 mM ammonium chloride (C), 12.5 μM bafilomycin A1 (D, left panel), 2 μM monensin (D, middle panel), and 15 μM chlorpromazine (D, right panel). β-Galactosidase activities for HHV-8 infectivity and luciferase activities for the pseudotyped retrovirus vectors on T1H6 cells were measured, and their means and standard deviations for %RLU from triplicate experiments are shown. 293T cells were infected with rSFVβgal (SFV) in the presence of 15 μM chlorpromazine, and the data are shown with those of HHV-8 (D, right panel).
Effects of HIV expression on HHV-8 replication.
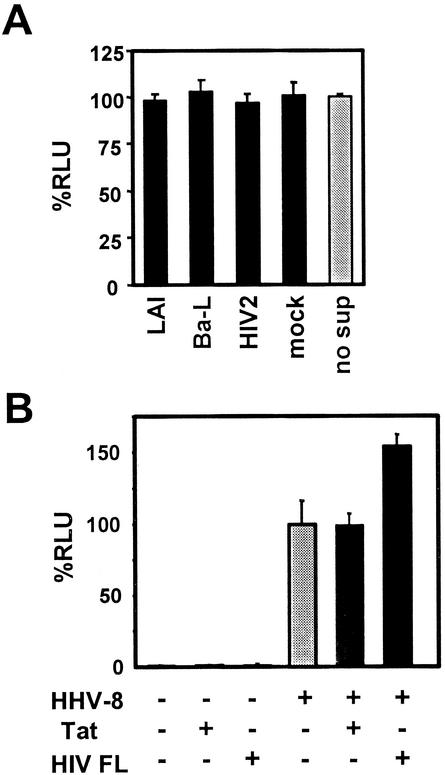
Several studies by others proposed potential effects of HIV infection with HHV-8 (24, 26, 37, 38, 52). However, these studies mainly focused on HHV-8 reactivation from latency in PEL cell lines. To characterize the HHV-8 entry mechanisms, here we asked whether HIV-encoded or -induced factors augment any of the early stages of HHV-8 infection, because HIV factors, such as Tat, may change the receptor localization or HHV-8 gene expression, for example, through stimulation of some signaling pathway (7, 8). First, the effects of soluble factors from HIV-infected cells on HHV-8 replication were analyzed. Conditioned media were prepared 0, 6, 24, and 48 h p.i. from cultures of the human T-cell line PM-1 (34) infected with prototype HIV strains. The cultures were tested for HIV-p24 antigen (Coulter Corporation, Miami, Fla.) 10 days p.i. to confirm the success of infection. T1H6 cells were incubated in the presence of these conditioned media for 4 or 24 h, beginning at 24 or 2 h before HHV-8 infection or at 2 h p.i. Treatment with any combination among the HIV strains, time points of harvesting the conditioned media, and timing of treatment of T1H6 cells with the media did not exhibit any detectable effect on HHV-8 infection (Fig. 4A). Then, we asked whether intracellular expression of HIV had any effect on HHV-8 infection. T1H6 cells were transfected with pSV-Tat72 (22) or with the HIV-1 proviral DNA clone pNL4-3 (2), infected with HHV-8 24 h after transfection, and then harvested 52 h p.i. We found that HIV-1 gene expression, but not Tat alone, enhanced HHV-8 replication slightly (Fig. 4B). A recent study reported that HIV infection activated the Rta promoter in a transient transfection assay (53) and that this activation was mediated by factors other than Tat. However, because we did not observe any detectable increase of lytic HHV-8 infection in PEL cells, including BCBL-1, JSC-1, and BC-3 cells, by HIV-1 infection (data not shown), further study is required to see whether increase of Rta expression by HIV-1 explains the slight effect that we observed in T1H6 cells. Studies on the factor(s) of HIV-1 that caused the HHV-8 activation in T1H6 cells are under way.
FIG. 4.
Interaction of HIV-1 with HHV-8. (A) Soluble factors from HIV-infected cells had no effect. Conditioned media were prepared from PM-1 cells infected with HIV-1LAI (LAI) (9), HIV-1Ba-L (Ba-L) (23), or HIV-2CDC77618 (HIV-2) (44) or from mock-infected cells, 24 h p.i. T1H6 cells were incubated in the presence of the conditioned media for 24 h and then infected with 0.5 μl of the HHV-8 stock described in the legend for Fig. 1D. The cells were harvested 52 h p.i. β-Galactosidase activities from triplicate experiments are shown as means and standard deviations for %RLU by using the activities obtained from the HHV-8-infected cells without addition of any conditioned media as a 100% control (no sup). (B) HIV-1 expression, but not Tat alone, slightly enhanced HHV-8 replication. T1H6 cells were transfected with vector plasmid pSV2, with pSV-Tat72 (Tat), and with pNL4-3 (HIV FL) and infected with HHV-8 1 day later. They were harvested 52 h p.i. β-Galactosidase activities from each condition are shown as means and standard deviations for %RLU by using the activities obtained from the HHV-8-infected cells that were transfected with pSV2 as a 100% control (shaded bar).
The reporter cell line that we developed in this study will be available for any noncommercial use upon written request.
Acknowledgments
We thank D. W. Russell, S. M. Owen, Y. Chang, R. F. Ambinder, and K. G. Kousoulas for pPAM3, HIV-2CDC77618, BC-1, JSC-1, and rabbit anti-HHV-8 serum, respectively. We also acknowledge L. T. Krug for sharing her data prior to publication and H. Folarin, M. Sharma, F. R. Stamey, and D. L. Rudolph for technical assistance. BCBL-1, pSV-Tat72, pNL4-3, HIV-1LAI, and HIV-1Ba-L, were obtained through the AIDS Research and Reference Reagent Program, NIH. We thank the contributors who made these materials available through the program.
REFERENCES
- 1.Ablashi, D. V., L. G. Chatlynne, J. E. Whitman, Jr., and E. Cesarman. 2002. Spectrum of Kaposi's sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clin. Microbiol. Rev. 15:439-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilar, J. S., M. Rice, and E. K. Wagner. 1999. The polysulfonated compound suramin blocks adsorption and lateral diffusion of herpes simplex virus type-1 in Vero cells. Virology 258:141-151. [DOI] [PubMed] [Google Scholar]
- 4.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akula, S. M., N. P. Pramod, F. -Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235-249. [DOI] [PubMed] [Google Scholar]
- 6.Akula, S. M., N. P. Pramod, F. -Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 7.Albini, A., R. Soldi, D. Giunciuglio, E. Giraudo, R. Benelli, L. Primo, D. Noonan, M. Salio, G. Camussi, W. Rockl, and F. Bussolino. 1996. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat. Med. 2:1371-1375. [DOI] [PubMed] [Google Scholar]
- 8.Barillari, G., R. Gendelman, R. C. Gallo, and B. Ensoli. 1993. The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc. Natl. Acad. Sci. USA 90:7941-7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barré-Sinoussi, F., J. C. Chermann, F. Rey, M. T. Nugeyre, S. Chamaret, J. Gruest, C. Dauguet, C. Axler-Blin, F. Vézinet-Brun, C. Rouzioux, W. Rozenbaum, and L. Montagnier. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868-871. [DOI] [PubMed] [Google Scholar]
- 10.Birkmann, A., K. Mahr, A. Ensser, S. Yǎgubǒglu, F. Titgemeyer, B. Fleckenstein, and F. Neipel. 2001. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J. Virol. 75:11583-11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boshoff, C., and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus: a new DNA tumor virus. Annu. Rev. Med. 52:453-470. [DOI] [PubMed] [Google Scholar]
- 12.Cannon, J. S., D. Ciufo, A. L. Hawkins, C. A. Griffin, M. J. Borowitz, G. S. Hayward, and R. F. Ambinder. 2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi's sarcoma herpesvirus-containing supernatant. J. Virol. 74:10187-10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cesarman, E., P. S. Moore, P. H. Rao, G. Inghirami, D. M. Knowles, and Y. Chang. 1995. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 86:2708-2714. [PubMed] [Google Scholar]
- 14.Chazal, N., G. Singer, C. Aiken, M. -L. Hammarskjöld, and D. Rekosh. 2001. Human immunodeficiency virus type 1 particles pseudotyped with envelope proteins that fuse at low pH no longer require Nef for optimal infectivity. J. Virol. 75:4014-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciufo, D. M., J. S. Cannon, L. J. Poole, F. Y. Wu, P. Murray, R. F. Ambinder, and G. S. Hayward. 2001. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 75:5614-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Compton, T., R. R. Nepomuceno, and D. M. Nowlin. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191:387-395. [DOI] [PubMed] [Google Scholar]
- 17.Deng, H., J. T. Chu, M. B. Rettig, O. Martinez-Maza, and R. Sun. 2002. Rta of the human herpesvirus 8/Kaposi sarcoma-associated herpesvirus up-regulates human interleukin-6 gene expression. Blood 100:1919-1921. [DOI] [PubMed] [Google Scholar]
- 18.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17:4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doxsey, S. J., F. M. Brodsky, G. S. Blank, and A. Helenius. 1987. Inhibition of endocytosis by anti-clathrin antibodies. Cell 50:453-463. [DOI] [PubMed] [Google Scholar]
- 20.Empig, C. J., and M. A. Goldsmith. 2002. Association of the caveola vesicular system with cellular entry by filoviruses. J. Virol. 76:5266-5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esty, A. 1991. Receptor-specific serum-free cell attachment using a highly stable engineered protein polymer. Am. Biotechnol. Lab. 9:44. [PubMed] [Google Scholar]
- 22.Frankel, A. D., and C. O. Pabo. 1988. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55:1189-1193. [DOI] [PubMed] [Google Scholar]
- 23.Gartner, S., P. Markovits, D. M. Markovitz, M. H. Kaplan, R. C. Gallo, and M. Popovic. 1986. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233:215-219. [DOI] [PubMed] [Google Scholar]
- 24.Harrington, W., Jr., L. Sieczkowski, C. Sosa, S. Chan-a-Sue, J. P. Cai, L. Cabral, and C. Wood. 1997. Activation of HHV-8 by HIV-1 tat. Lancet 349:774-775. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 26.Huang, L. M., M. F. Chao, M. Y. Chen, H. Shih, Y. P. Chiang, C. Y. Chuang, and C. Y. Lee. 2001. Reciprocal regulatory interaction between human herpesvirus 8 and human immunodeficiency virus type 1. J. Biol. Chem. 276:13427-13432. [DOI] [PubMed] [Google Scholar]
- 27.Hudson, J. B. 1988. Further studies on the mechanism of centrifugal enhancement of cytomegalovirus infectivity. J. Virol. Methods 19:97-108. [DOI] [PubMed] [Google Scholar]
- 28.Inoue, N., E. C. Mar, S. C. Dollard, C. P. Pau, Q. Zheng, and P. E. Pellett. 2000. New immunofluorescence assays for detection of human herpesvirus 8-specific antibodies. Clin. Diagn. Lab. Immunol. 7:427-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue, N., and D. W. Russell. 1998. Packaging cells based on inducible gene amplification for the production of adeno-associated virus vectors. J. Virol. 72:7024-7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juhasz, A., J. Konya, Z. Beck, E. Remenyik, G. Veress, A. Begany, I. Medgyessy, J. Hunyadi, and L. Gergely. 2001. HHV-8 ELISA based on a one-step affinity capture of biotinylated K8.1 antigen. J. Virol. Methods 94:163-172. [DOI] [PubMed] [Google Scholar]
- 31.Klein, G., T. Lindahl, M. Jondal, W. Leibold, J. Menezes, K. Nilsson, and C. Sundstrom. 1974. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc. Natl. Acad. Sci. USA 71:3283-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 76:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukac, D. M., R. Renne, J. R. Kirshner, and D. Ganem. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252:304-312. [DOI] [PubMed] [Google Scholar]
- 34.Lusso, P., F. Cocchi, C. Balotta, P. D. Markham, A. Louie, P. Farci, R. Pal, R. C. Gallo, and M. S. Reitz, Jr. 1995. Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J. Virol. 69:3712-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsh, M., and R. Bron. 1997. SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J. Cell Sci. 110: 95-103. [DOI] [PubMed] [Google Scholar]
- 36.McClure, M. O., M. A. Sommerfelt, M. Marsh, and R. A. Weiss. 1990. The pH independence of mammalian retrovirus infection. J. Gen. Virol. 71:767-773. [DOI] [PubMed] [Google Scholar]
- 37.Merat, R., A. Amara, C. Lebbe, H. de The, P. Morel, and A. Saib. 2002. HIV-1 infection of primary effusion lymphoma cell line triggers Kaposi's sarcoma-associated herpesvirus (KSHV) reactivation. Int. J. Cancer 97:791-795. [DOI] [PubMed] [Google Scholar]
- 38.Mercader, M., B. Taddeo, J. R. Panella, B. Chandran, B. J. Nickoloff, and K. E. Foreman. 2000. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am. J. Pathol. 156:1961-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, A. D., and C. Buttimore. 1986. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol. Cell. Biol. 6:2895-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, N., and L. M. Hutt-Fletcher. 1992. Epstein-Barr virus enters B cells and epithelial cells by different routes. J. Virol. 66:3409-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-ζ-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 77:1524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Leary, J., M. Kennedy, D. Howells, I. Silva, V. Uhlmann, K. Luttich, S. Biddolph, S. Lucas, J. Russell, N. Bermingham, M. O'Donovan, M. Ring, C. Kenny, M. Sweeney, O. Sheils, C. Martin, S. Picton, and K. Gatter. 2000. Cellular localisation of HHV-8 in Castleman's disease: is there a link with lymph node vascularity? Mol. Pathol. 53:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Owen, S. M., D. Ellenberger, M. Rayfield, S. Wiktor, P. Michel, M. H. Grieco, F. Gao, B. H. Hahn, and R. B. Lal. 1998. Genetically divergent strains of human immunodeficiency virus type 2 use multiple coreceptors for viral entry. J. Virol. 72:5425-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 47.Roan, F., N. Inoue, and M. K. Offermann. 2002. Activation of cellular and heterologous promoters by the human herpesvirus 8 replication and transcription activator. Virology 301:293-304. [DOI] [PubMed] [Google Scholar]
- 48.Sharkey, C. M., C. L. North, R. J. Kuhn, and D. A. Sanders. 2001. Ross River virus glycoprotein-pseudotyped retroviruses and stable cell lines for their production. J. Virol. 75:2653-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song, M. J., H. J. Brown, T. T. Wu, and R. Sun. 2001. Transcription activation of polyadenylated nuclear RNA by Rta in human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:3129-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stamey, F. R., M. M. Patel, B. P. Holloway, and P. E. Pellett. 2001. Quantitative, fluorogenic probe PCR assay for detection of human herpesvirus 8 DNA in clinical specimens. J. Clin. Microbiol. 39:3537-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varthakavi, V., P. J. Browning, and P. Spearman. 1999. Human immunodeficiency virus replication in a primary effusion lymphoma cell line stimulates lytic-phase replication of Kaposi's sarcoma-associated herpesvirus. J. Virol. 73:10329-10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varthakavi, V., R. M. Smith, H. Deng, R. Sun, and P. Spearman. 2002. Human immunodeficiency virus type-1 activates lytic cycle replication of Kaposi's sarcoma-associated herpesvirus through induction of KSHV Rta. Virology 297:270-280. [DOI] [PubMed] [Google Scholar]
- 54.Wang, F. Z., S. M. Akula, N. P. Pramod, L. Zeng, and B. Chandran. 2001. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J. Virol. 75:7517-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wittels, M., and P. G. Spear. 1990. Penetration of cells by herpes simplex virus does not require a low pH-dependent endocytic pathway. Virus Res. 18:271-290. [DOI] [PubMed] [Google Scholar]