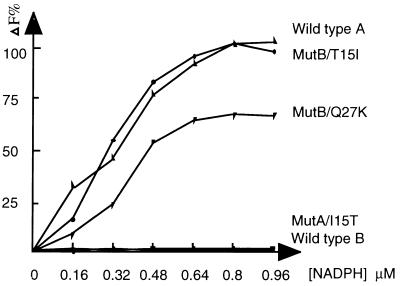
Figure 4.
Measurement of binding constants of NADPH to wild-type CR/20β-HSDs and mutants. The binding of NADPH to enzymes was determined by measuring the decrease in fluorescence emission at 336 nm (excitation wavelength of 290 nm) in 20 mM Tris⋅HCl/1 mM EDTA buffer (pH 8.0). Wild-type and mutant proteins (20 μg) were used, and the data are plotted as percentage of change of fluorescence against NADPH concentration, with maximum change in fluorescence of wild-type A defined as 100%.