Abstract
The human cytomegalovirus UL97 protein is an unusual protein kinase that is able to autophosphorylate and to phosphorylate certain exogenous substrates, including nucleoside analogs such as ganciclovir. However, no natural substrate of UL97 in infected cells has been identified. We report here that recombinant UL44 protein became radiolabeled when incubated with recombinant UL97 and [32P]ATP and that both proteins could be coimmunoprecipitated by an antibody that recognizes either protein. Subsequent studies showed that highly purified, recombinant UL97 phosphorylated purified, recombinant UL44. This phosphorylation occurred on serine and threonine residues and was sensitive to inhibition by maribavir and to a mutation that inactivates UL97 catalytic activity. Two-dimensional gel electrophoresis revealed the absence of specific phosphorylated forms of UL44 in immunoprecipitates from lysates of cells infected with a UL97 null mutant virus or with wild-type virus in the presence of maribavir. The results indicate that UL97 is sufficient to phosphorylate UL44 in vitro and is necessary for the normal phosphorylation of UL44 in infected cells. This strongly suggests that UL44 is a natural substrate of UL97.
Protein kinases play a crucial role in the regulation of protein function through phosphorylation of targeted proteins. Human cytomegalovirus (HCMV) encodes many phosphorylated proteins and at least one protein kinase, the product of the UL97 open reading frame (ORF) (6, 13). UL97 is most closely related in sequence to certain protein kinases encoded by various herpesviruses, which collectively form the HvUL protein kinase family (18). These enzymes share many motifs typical of protein kinases but are relatively divergent. The protein kinase motifs of UL97 are even more divergent than those of other family members (6). Biochemically, UL97 is rather unusual in that it can phosphorylate nucleoside analogs such as the antiviral drugs acyclovir and ganciclovir (30). Indeed, phosphorylation of ganciclovir by UL97 is responsible for much of the antiviral selectivity of this drug (29). In vitro, UL97 can autophosphorylate (1, 13). It can also phosphorylate certain exogenous protein and peptide substrates (1, 2, 8, 13; P. B. Sethna, M. G. Davis, C. L. Talarico, W. H. Miller, K. Blackburn, W. A. Burhart, M. B. Moyer, K. K. Biron, and R. J. Harvey, submitted for publication). With these more conventional substrates, it is rather specific. For example, it phosphorylates histones only on particular serine residues (2), but it does not phosphorylate various other proteins, for example, glutathione S-transferase (GST) (8) or various serine/threonine containing peptides (2). Its specificity appears to be due in part to an unusual preference for basic residues five positions downstream of the phosphorylation site (1, 2).
UL97 is important, but not completely essential, for viral replication (25). It has been reported to have roles at the stages of DNA replication, DNA encapsidation, and/or nuclear egress (3, 15, 33). An understanding of how UL97 functions during viral replication would be greatly aided by identifying its natural substrates. An initial step in that direction made use of maribavir (also known as 1263W94), a potent inhibitor of HCMV replication in tissue culture that acts via inhibition of UL97 (3). When lysates from wild-type (wt) HCMV-infected cells were incubated with radiolabeled ATP, the inclusion of maribavir reduced the phosphorylation of several proteins; the phosphorylation of these proteins was not reduced by maribavir in lysates from cells infected with a maribavir-resistant mutant (Sethna et al., submitted). One of these proteins was UL44, a phosphoprotein encoded by the HCMV UL44 ORF, also known as pp52 and ICP36 (5, 11, 16). UL44 is essential for HCMV DNA replication and appears to serve as the processivity subunit of the viral DNA polymerase (10, 24, 28, 32, 35), which might be relevant to the role of UL97 in viral infection. However, it was not shown that UL97 directly phosphorylates UL44, nor has there been evidence that UL97 is required for the normal phosphorylation of any viral protein in infected cells. We therefore sought to determine if UL44 is a substrate for UL97 in vitro and in infected cells.
MATERIALS AND METHODS
Reagents.
All reagents were from Sigma unless otherwise specified.
Cells and viruses.
Spodoptera frugiperda 9 cells, obtained from the American Type Culture Collection, were maintained in Grace's supplemented insect medium (Gibco BRL, Grand Island, N.Y.) with 10% fetal bovine serum (FBS) in 1-liter spinner flasks at 27°C. The cells were subcultured every 2 to 4 days. Recombinant baculoviruses expressing wt or mutant K355Q GST-UL97 proteins (13) were propagated and maintained by standard methods (9). Briefly, viral stocks were generated by infecting S. frugiperda 9 cells in cell culture dishes at a multiplicity of infection (MOI) of approximately 0.01 PFU/cell. After 5 days, the supernatants were harvested, clarified, and stored at 4°C in the dark. Titers of viral stocks were determined by plaque assay, and the stocks were used at an MOI of ca. 3 to 10.
Human foreskin fibroblast (HFF) cells were obtained from the American Type Culture Collection and grown in Dulbecco's modified Eagle's medium (DMEM) (Clontech) supplemented with 10% FBS. Cells were subcultured every 2 to 3 days and screened for mycoplasma once a month by PCR. HCMV wt strain AD169 was obtained from the American Type Culture Collection. RCΔ97, a UL97 mutant isolate derived from AD169, which contains an insertion including the Escherichia coli lacZ and gpt genes replacing most of UL97 (25), was generously provided by Mark Prichard. The isolate used here was RCΔ97.08; it was plaque purified, and stocks were screened for the presence of plaques that failed to express β-galactosidase. Viral stocks were prepared and titers were determined as described previously (31).
Expression and purification of UL97.
wt GST-UL97 and K355Q GST-UL97 were expressed from baculovirus vectors as previously described (13). Initial studies were performed with protein purified as previously described (13). For subsequent studies, based on our studies of UL44 phosphorylation (see below), the purification protocol was modified as follows. The protein that eluted from a glutathione Sepharose column was bound to a Q-Sepharose column (all column materials were from Amersham Pharmacia Biotech) that was previously equilibrated with elution buffer (EB) (50 mM Tris [pH 8.0], 2 mM dithiothreitol [DTT], 2 mM EDTA, 10% glycerol) with 50 mM NaCl (EB-50). The Q-Sepharose was washed with 30 column volumes of EB-50 and 30 column volumes of EB-150, and protein was then eluted with EB-500. This material was loaded onto a phenyl-Sepharose column equilibrated with EB-1,000, and the column was washed with 30 column volumes of EB-500 and 10 volumes of EB-300 with 0.1% Triton X-100. GST-UL97 was then eluted with EB-0 with 0.1% Triton X-100, concentrated with a Centricon-30 column, and dialyzed for 2 h against EB-50. This procedural modification resulted in protein that is apparently homogeneous as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining (similar to that published previously [13]). The concentrations of GST-UL97 protein were determined by amino acid analysis performed by Angelo Dickerson at the Molecular Biology Core Facility of the Dana-Farber Cancer Institute. Purified preparations were stored at −80°C.
Expression and purification of His6-UL44 and GST-UL44.
pRSET44, a generous gift from P. Ertl (GlaxoSmithKline), contains the UL44 ORF linked to sequences encoding a tag of six histidine residues (His6-UL44). Plasmid pD15-UL44, generously supplied by Brent Appleton (Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School), encodes UL44 fused to GST (GST-UL44) with the two proteins separated by a PreScission protease cleavage site. These plasmids were transformed into BL21 DE3 pLysS (Novagen). Cultures were grown in Luria broth with 50 μg of ampicillin per ml to an optical density at 600 nm of ∼0.5 and induced with 1 mM isopropyl-β-d-thiogalactopyranoside for 3 h at 37°C for pRSET44 and at 30°C for pD15-UL44. The cells were pelleted (washed with phosphate-buffered saline in the case of GST-UL44), frozen, and stored at −80°C.
In the case of His6-UL44, the pellet was suspended in 50 mM HEPES (pH 7.5)-2 mM DTT-1 mM EDTA-500 mM NaCl (HEPES buffer). The suspensions were sonicated by using a 50% duty cycle of three 30-s pulses, with 30 s to cool between pulses, and the lysates were clarified at 18,000 × g for 45 min at 4°C. The cleared lysate was diluted 10-fold with HEPES buffer without NaCl. This was bound to SP-Sepharose, the column was washed with 7 column volumes of HEPES buffer at 50 mM NaCl, and the protein was eluted with an NaCl gradient (50 mM to 1 M) in HEPES buffer with 10% glycerol. (SP-Sepharose was used instead of nickel because the protein tended to aggregate in the absence of DTT, which is incompatible with nickel column chromatography.) His6-UL44 eluted at ∼400 mM NaCl and was frozen in aliquots at −20°C. The protein was quantified by using the Bio-Rad protein reagent.
In the case of GST-UL44, the pellet was thawed and suspended in EB-500 lacking EDTA but containing 1% Triton X-100, 5 mM MgCl2, complete protease inhibitors, and ∼0.5 μg of DNase I (Roche) per ml. The suspensions were sonicated, and lysates were clarified as described above. The clarified lysate was loaded onto a 5-ml glutathione-Sepharose column. The beads were washed with 100 ml of EB-500 containing complete protease inhibitors and with 100 ml of EB-500. The fusion protein was eluted with EB-500 plus 15 mM glutathione, mixed with 0.75 μl of Prescission protease (Amersham Pharmacia Biotech) per ml of eluant, and dialyzed overnight against EB-150. The eluant from the glutathione-Sepharose was bound to denatured, single-stranded DNA agarose previously equilibrated with EB-150. Unbound proteins were removed by extensively washing the DNA agarose with EB-150, and UL44 was eluted with EB-500. This eluant was then repeatedly passed through a 1-ml glutathione-Sepharose column equilibrated with EB-500 to reduce GST-containing contaminants. Protein was concentrated with a Centricon column and quantified by amino acid analysis.
Antibodies.
The antibodies used were anti-UL44 monoclonal antibodies 28-21 (kindly provided by William Britt, Department of Pediatrics, University of Alabama at Birmingham) and M612460 (Fitzgerald), anti-UL83 monoclonal antibody 28-19 (also kindly provided by William Britt), and an anti-UL97 antibody that was generated by a commercial supplier (Zenica) following immunization of New Zealand White rabbits with two synthetic peptides derived from UL97 with Freund’s complete adjuvant. The peptides were Asp-Ala-Ala-Ser-Asp-Lys-Glu-Asn-Leu-Arg-Arg-Pro-Val-Cys (residues 118 to 131 of UL97) and Ala-Ala-Ser-Gly-Asp-Gly-Tyr-His-Gly-Leu-Arg-Cys (residues 139 to 150).
Coimmunoprecipitation.
His6-UL44 was dephosphorylated with protein phosphatase 1 (New England BioLabs) according to the manufacture's recommendations, and the reaction was terminated with a 1,000-fold molar excess of protein phosphatase inhibitor 2 (New England BioLabs). Dephosphorylated His6-UL44 (4 μg) and GST-UL97 (192 ng), purified as described previously (13), were incubated together in a buffer composed of 50 mM sodium 2-(N-cyclohexylamino)ethanesulfonic acid (pH 9.5), 10 mM MgCl2, 1 M NaCl, 2 μM ATP, 2 mM DTT, 1 mM sodium orthovanadate, 1 mM NaF, and 0.033 μM [γ-33P]ATP for 90 min at 37°C. Aliquots (75 μl) were diluted into 1 ml of radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS, and 1% deoxycholate) and incubated on ice for 30 min. Selected diluted aliquots of the kinase reaction mixture were mixed with either 500 μl of anti-UL44 monoclonal antibody 28-21, 5 μl of the anti-UL97 antibody, or 500 μl of the anti-UL83 monoclonal antibody and incubated at 4°C for 16 h. with mixing. Fifty microliters of a 50% slurry of protein A-Sepharose (Roche) was then added, and mixing was continued for an additional hour. The beads were washed twice with RIPA buffer and once with RIPA buffer without detergent. Beads were suspended in SDS-PAGE loading buffer and boiled. The supernatants were separated by SDS-4 to 10% PAGE, the gel was dried, and the radioactivity was detected with a phosphorimager.
Protein kinase assays.
Each reaction mixture contained 20 to 25 ng of purified GST-UL97, 0.5 μg of UL44, 50 mM Tris (pH 9.5), 20 μM ATP, 300 mM NaCl, 1 mM DTT, 10 mM MgCl2, 0.5 μl of [γ-32P]ATP (3,000 to 6,000 Ci/mmol) (Dupont NEN), and, in certain experiments, selected concentrations of maribavir in a total volume of 10 μl. The final NaCl concentration contributed by the enzymes was <50 mM. The reaction mixtures were incubated at 37°C for 30 min, and the reactions were terminated by the addition of concentrated SDS-PAGE loading buffer. The samples were heated to 100°C for 3 to 5 min and separated by SDS-10% PAGE. The gel was dried onto blotting paper under vacuum, and incorporated radioactivity was monitored with a phosphorimager (Molecular Imager FX System; Bio-Rad). For analysis of the effects of maribavir, the data were fitted to the Hill equation by using SigmaPlot.
Phosphoamino acid analysis.
UL44 (10 μg) was phosphorylated in a 100-μl reaction mixture with GST-UL97 (800 ng), 50 mM Tris (pH 9.5), 20 μM ATP, 10 mM MgCl2, 1 mM DTT, and ∼50 μCi of [γ-32P]ATP. The reaction mixture was incubated at 37°C for 2 h, and the reaction was terminated by the addition of SDS-PAGE loading buffer. The solution was reduced to ∼20 μl under vacuum, separated by 10% SDS-PAGE, and transferred to an Immobilon-P polyvinylidene difluoride membrane (Millipore). The membrane was extensively washed with 50 mM NH4HCO3. The UL44 band was identified by autoradiography, cut from the membrane, and incubated for 3 h at 37°C with 1 μg of trypsin (sequencing grade; Roche) in 50 μl of 50 mM NH4HCO3. The resulting peptides were hydrolyzed by the addition of 50 μl of 12 M HCl at 95°C for 90 min. The supernatant, containing the released amino acids, was transferred to a microcentrifuge tube, and the liquid was evaporated under vacuum. The amino acids were suspended in water, mixed with standards, and spotted on a cellulose thin-layer chromatography plate (EM Science). Semidry thin-layer electrophoresis was performed at 1,000 V for 2 h in 1% pyridine with acetic acid to pH 3.0 with a Multiphor II instrument (Amersham Pharmacia). Standards were visualized with ninhydrin, and labeled amino acids were detected with a Molecular Imager FX.
Analysis of phosphorylated forms of UL44 in infected cells.
HFF cells were infected at an MOI of 1 PFU/cell (inoculum titers were confirmed by back titration) in DMEM with 5% FBS for 2 h. Inocula were removed, and the cells were washed two times with DMEM plus 5% FBS. DMEM plus 5% FBS, either with or without 1 μM maribavir, was then added, and incubation was continued at 37°C. At 3 days postinoculation (p.i.), cells were rinsed twice with DMEM-1% FBS-glutamine without inorganic phosphate and were then incubated in 2 ml of that medium containing 1 mCi of [32P]orthophosphate for 2 h. The medium was removed, and the cells were rinsed twice in ice-cold Tris-buffered saline (TBS) (20 mM Tris HCl [pH 7.5], 150 mM NaCl) and then scraped into ice-cold TBS. Cells were pelleted by low-speed centrifugation and resuspended in 100 μl of buffer containing 50 mM Tris HCl (pH 8), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM NaF, and complete protease inhibitors. After 30 min on ice, the suspension was clarified by centrifugation at 12,000 × g at 4°C for 10 min. A 10-μl aliquot was reserved for immunoblotting. The remaining lysate was diluted with 1 ml of NET-gel (50 mM Tris [pH 7.5], 150 mM NaCl, 0.1% NP-40, 1 mM EDTA, 0.25% gelatin, 50 mM NaF, and complete protease inhibitors), and 10 μl of anti-UL44 monoclonal antibody M612460 was added. Following incubation with shaking at 4°C for 1 h, 50 μl of a 50% slurry of protein G-Sepharose was added and incubation with shaking at 4°C was continued for 1 h. The Sepharose was pelleted by centrifugation and washed twice (15 min per wash) with NET-gel and once with TBS. Proteins were eluted from the Sepharose with a buffer containing 8 M urea, 2 M thiourea, 0.1% Triton-X-100, 1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, and 10 mM Tris base, and the eluant was collected following centrifugation.
The proteins were then analyzed by two-dimensional gel electrophoresis. The first dimension was isoelectric focusing (IEF) using Immobiline dry strips with a pH gradient of 6 to 11 (Amersham Pharmacia Biotech), performed using the IPGphor system (Amersham Pharmacia Biotech) according to the manufacturer's instructions, with modifications. Briefly, 250 μl of freshly prepared rehydration buffer (7 M urea, 2 M thiourea, 1.6% Triton X-100, 2% CHAPS, 0.5% IPG buffer [Amersham Pharmacia Biotech], and 18 mM DTT, plus bromophenol blue) was mixed with the sample. Strips were rehydrated for 15 h at room temperature. IEF was performed at 500 V (3 h), 1,000 V (3 h), 4,000 V (1 h), 6,000 V (1 h), and 8,000 V (11 h) at <50 mA per strip. After IEF, the strips were equilibrated with 10 ml of freshly prepared SDS equilibration buffer (50 mM Tris HCl, 6 M urea, 30% glycerol, 2% SDS, and 64 mM DTT, plus bromophenol blue), and the proteins were electrophoresed alongside prestained molecular weight markers (Invitrogen) on SDS-4 to 20% polyacrylamide gels (16 by 18 by 0.75 cm; Jule). The gels were dried, and the radiolabeled proteins were visualized by autoradiography.
RESULTS
Recombinant UL44 fusion protein coimmunoprecipitates with GST-UL97 and is phosphorylated.
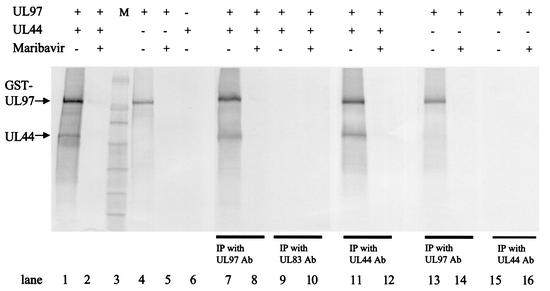
Experiments using lysates of HCMV-infected cells suggested that UL44 could be a substrate for UL97 (Sethna et al., submitted). As an initial step in determining whether UL97 could directly phosphorylate UL44, coimmunoprecipitation of the two proteins was undertaken following incubation with radiolabeled ATP. UL44 was expressed in E. coli as a His6 fusion protein and isolated by using SP-Sepharose, and GST-UL97 was expressed in a baculovirus system and purified as previously described (13). The two proteins were incubated together with radiolabeled ATP in a protein kinase assay and then were immunoprecipitated with either an anti-UL97 antibody, an anti-UL44 antibody, or, as a control, an antibody against HCMV UL83. Both fusion proteins were found to be radiolabeled prior to immunoprecipitation (Fig. 1, lane 1 [minor species other than GST-UL97 and UL44 likely are proteolytic fragments of these proteins]). Following immunoprecipitation with either anti-UL97 or anti-UL44 antibody, a substantial fraction of each labeled protein was found in the immunoprecipitate (lanes 7 and 11). However, no labeled proteins were found following immunoprecipitation with the control antibody against UL83 (lane 9). GST-UL97 alone, as previously reported (13), autophosphorylated, with little or no labeling at the position of UL44 (lane 4). It was precipitated by the anti-UL97 antibody (lane 13) but was not precipitated by the anti-UL44 antibody in the absence of UL44 (lane 15). When UL44 was incubated with radiolabeled ATP, no incorporation of label was observed (lane 6).
FIG. 1.
Coimmunoprecipitation and phosphorylation of UL44 and UL97. GST-UL97 (UL97) and His6-UL44 (UL44) were incubated alone or together, as indicated, in kinase buffer in the presence of radiolabeled ATP, in either the presence or absence of 1 μM maribavir. Aliquots of the reaction mixtures were resolved by SDS-PAGE and analyzed with a phosphorimager (lanes 1 to 6). The remaining portions of the reactions were precipitated (IP) (lanes 7 to 16) by the addition of either a UL97-specific antibody (Ab) (lanes 7, 8, 13, and 14), a UL83-specific antibody as a negative control (lanes 9 and 10), or a UL44-specific antibody (lanes 11, 12, 15, and 16), followed by protein A-Sepharose, and resolved by SDS-PAGE and analyzed with a phosphorimager. M, molecular weight markers. Approximately 50% more of each immunoprecipitate was analyzed than the corresponding sample analyzed prior to immunoprecipitation.
The fact that both proteins were labeled when GST-UL97 was mixed with UL44 suggested that UL44 was a substrate for UL97. To examine the specificity of labeling further, the effect of a specific inhibitor of UL97, maribavir (also known as 1263W94) (1, 3) was examined. Maribavir at 1 μM greatly reduced phosphorylation of both proteins or of UL97 alone, either before or after immunoprecipitation (Fig. 1, lanes 2, 5, 8, 12, and 14). Thus, these initial results were consistent with the hypothesis that UL44 is a substrate for UL97, at least in vitro.
Phosphorylation of UL44 with purified proteins.
To test further whether UL44 is a substrate for UL97, we performed studies using more highly purified proteins and also made use of a catalytically inactive, mutant form of UL97, GST-UL97 K355Q (13), in which a highly conserved lysine residue (12) is replaced with glutamine. The mutant protein serves as a control in the purification of GST-UL97, because if it exhibits kinase activity, that strongly suggests the presence of a contaminating kinase. When the original GST-UL97 purification scheme was followed (13), purified GST-UL97 K355Q was still able to phosphorylate His6-UL44 (data not shown). To eliminate the contaminant, the purification scheme was modified to include a high-salt wash step during anion-exchange chromatography and a hydrophobic interaction chromatography step as detailed in Materials and Methods.
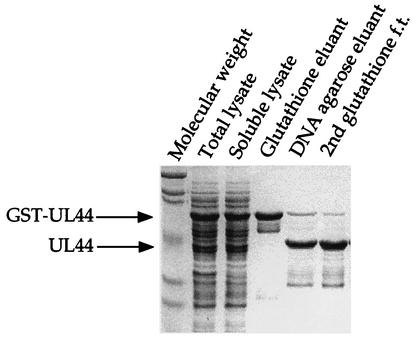
Analysis of UL44 phosphorylation was further complicated because the original expression construct, which used the pRSET vector, added an additional 36 amino acids to the N terminus of UL44, including several serine residues and a threonine residue. It was possible that the phosphorylation of His6-UL44 observed in the immunoprecipitation assay resulted from the phosphorylation of one of these tag residues. Although there is a site for cleavage by enterokinase present in His6-UL44, attempts to remove the tag by using this enzyme resulted in degraded protein. We therefore made use of an expression plasmid in which UL44 is expressed as a fusion to GST with a PreScission protease cleavage site separating the fusion partners. This recombinant protein was purified by using glutathione-Sepharose and was cleaved to release the fusion partner, leaving only four residues (Gly-Pro-Leu-Glu) on the N terminus of UL44. UL44 was purified with single-stranded DNA agarose, and the amount of uncleaved GST-UL44 was reduced by passing the preparation over a second glutathione-Sepharose column (Fig. 2). The final preparation was ∼90% pure, with the only visible contaminants being a small amount of uncleaved GST-UL44 and a spectrum of smaller proteins that retain the ability to bind DNA agarose. These smaller proteins are most likely proteolytic fragments of UL44. The UL44 prepared from the GST fusion construct was used for all subsequent in vitro experiments.
FIG. 2.
Purification of UL44. GST-UL44 was expressed in E. coli, the E. coli was lysed, and GST-UL44 was purified initially as a fusion protein, followed by cleavage of the fusion partner, permitting purification of UL44 as detailed in Materials and Methods. Aliquots of the total lysate, the lysate following centrifugation (Soluble lysate), the protein purified on glutathione-Sepharose (Glutathione eluant), the cleaved protein purified on DNA agarose (DNA agarose eluant), and the protein that flowed through a glutathione-Sepharose column (glutathione f.t.) were resolved by SDS-PAGE and detected by Coomassie blue staining. The positions of GST-UL44 and UL44 are indicated to the left.
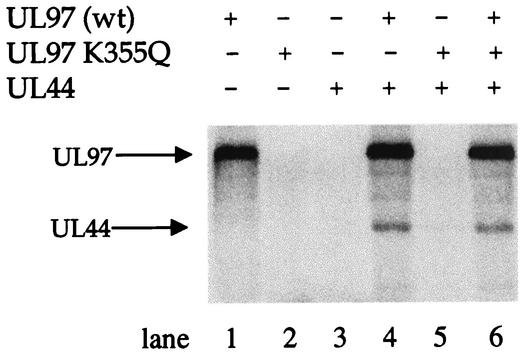
We then tested whether this more highly purified preparation of UL44 could serve as a substrate for the more highly purified GST-UL97 prepared with the modified purification protocol. Indeed, the highly purified GST-UL97 phosphorylated UL44 (Fig. 3, lane 4). In contrast, GST-UL97 K355Q, expressed and purified under identical conditions, was unable to phosphorylate UL44 or itself (Fig. 3, lane 5). Purified UL44 alone did not become phosphorylated (Fig. 3, lane 3), and the GST-UL97 K355Q preparation did not inhibit phosphorylation of UL44 by wt GST-UL97 (Fig. 3, lane 6). Thus, phosphorylation of UL44 was sensitive to the K355Q mutation.
FIG. 3.
In vitro phosphorylation of UL44 by UL97. Recombinant, purified HCMV GST-UL97, GST-UL97 K355Q, and UL44 were incubated alone or together, as indicated, in kinase buffer in the presence of radiolabeled ATP. The proteins were resolved by SDS-PAGE and analyzed with a phosphorimager. The positions of the GST-UL97 and UL44 proteins are indicated to the left gel.
Inhibition of UL44 phosphorylation by maribavir.
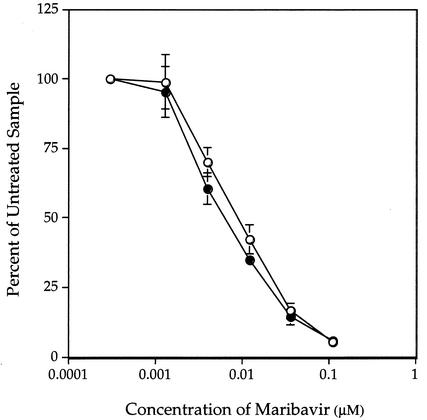
Maribavir is a specific inhibitor of UL97 autophosphorylation and of its phosphorylation of histones and synthetic peptides in vitro (1-3). It also reduced the phosphorylation of UL44 in an infected-cell lysate-based assay (Sethna et al., submitted) and in coimmunoprecipitates of UL97 and UL44 (Fig. 1). To determine the effect of maribavir with more highly purified proteins, GST-UL97 and UL44 were incubated together in kinase buffer containing [γ-32P]ATP and selected concentrations of maribavir. Quantification of phosphate incorporation clearly showed a dose-dependent inhibition of both autophosphorylation and UL44 phosphorylation (Fig. 4). The dose-response curves were nearly superimposable, with very similar concentrations of maribavir (∼60 nM) required for 50% inhibition of autophosphorylation and of UL44 phosphorylation. These pharmacological data and the mutational data reported above provide strong evidence that UL97 can directly phosphorylate UL44.
FIG. 4.
Inhibition of UL97 autophosphorylation (○) and the phosphorylation of UL44 (•) by maribavir. Selected concentrations of maribavir were included in an in vitro protein kinase assay, and the proteins were separated by SDS-PAGE. The relative amounts of radioactivity incorporated into GST-UL97 and into UL44 were quantified with a phosphorimager and plotted as the percentage of incorporation at each drug concentration relative to that in the absence of drug. Error bars indicate standard deviations.
Phosphoamino acid analysis.
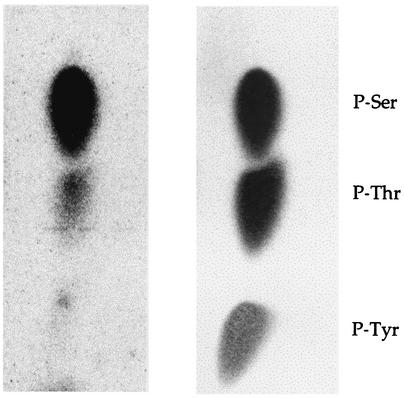
UL97 is known to autophosphorylate on serines and threonines (1, 13) and to phosphorylate histone H2B on serine residues (2). The sequence of UL44 predicts 38 serine, 30 threonine, and 10 tyrosine residues. To identify the amino acid(s) of UL44 phosphorylated by GST-UL97, 32P-labeled UL44 was hydrolyzed, and the resulting amino acid mixture was separated by thin-layer electrophoresis. Autoradiography of the cellulose plate showed a near-perfect alignment of the radioactivity with ninhydrin-stained standards of phosphoserine and phosphothreonine, but not phosphotyrosine (Fig. 5). There was roughly 10 times more radioactivity associated with phosphoserine than with phosphothreonine. Thus, UL97 phosphorylates UL44 mainly on serine residues.
FIG. 5.
Phosphoamino acid analysis of phosphorylated UL44. UL44 was phosphorylated in vitro, separated from GST-UL97 by SDS-PAGE, and hydrolyzed with 6 M HCl. The released amino acids were recovered, spotted onto a cellulose thin-layer plate, and separated by semidry electrophoresis. The plate was stained with ninhydrin and scanned (right panel), and labeled amino acids were analyzed with a phosphorimager (left panel). The positions of the ninhydrin-stained phosphoserine (P-Ser), phosphothreonine (P-Thr), and phosphotyrosine (P-Tyr) standards are indicated to the right.
Inhibition of UL97 alters UL44 phosphorylation in infected cells.
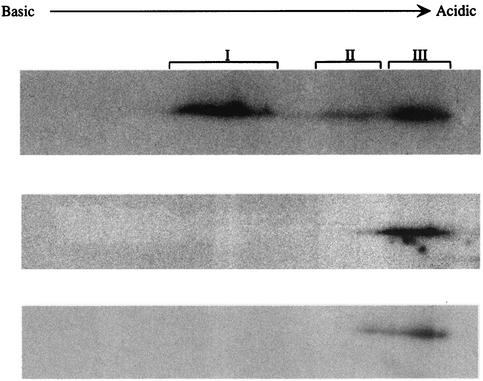
Our results to this point indicated that UL97 is sufficient to phosphorylate UL44 in vitro but did not address whether UL97 has a role in UL44 phosphorylation in HCMV-infected cells. We therefore infected HFF cells with wild-type HCMV strain AD169, in either the presence or absence of maribavir, or with RCΔ97, a UL97 null mutant (25) At 3 days p.i., the cells were pulse-labeled with [32P]orthophosphate, and cell lysates were prepared. UL44 was immunoprecipitated from the lysates with a monoclonal antibody, and the immunoprecipitated proteins were resolved by two-dimensional gel electrophoresis. The radiolabeled UL44 (confirmed by mass spectroscopic analysis [data not shown]) from cells infected with wt virus in the absence of maribavir migrated as a series of species with different isoelectric points (pI), with most of the radioactivity in three regions of the gel; these were designated I, II, and III in order of decreasing pI, with regions I and III being most intensely labeled (Fig. 6, top panel). However, in gels of samples from maribavir-treated cells or from UL97 null mutant-infected cells, most of the radioactivity was only in region III, even after long exposure of the gels to film (Fig. 6, bottom two panels). Thus, either pharmacological inhibition or genetic elimination of UL97 resulted in altered phosphorylation of UL44; i.e., UL97 is required for normal phosphorylation of UL44 in HCMV-infected cells.
FIG. 6.
Altered phosphorylation of UL44 in the absence of functional UL97. 32P-labeled UL44 was immunoprecipitated from HFF cells infected with wt AD169 in the absence of drug (top panel), with RCΔ97 (middle panel), or with AD169 in the presence of 1 μM maribavir (bottom panel) and resolved by two-dimensional gel electrophoresis, The radioactive species were detected by autoradiography. Film exposure times for the lower two panels were longer than that for the top panel.
DISCUSSION
HCMV UL97 has been known for some time as a protein kinase that can phosphorylate an antiviral nucleoside analog, ganciclovir (13, 17, 29, 30), but its natural substrates have remained unknown. We show here that HCMV UL44, an accessory subunit of HCMV DNA polymerase (10, 32), can serve as a substrate for purified UL97 in vitro and that its phosphorylation in infected cells is altered by a specific UL97 inhibitor or during infection with a UL97 mutant virus. These results strongly suggest that UL44 is a natural substrate of UL97.
Phosphorylation of exogenous viral proteins in vitro.
Several protein substrates of UL97 in vitro have been identified previously. One of these is UL97 itself, which autophosphorylates residues in its N-terminal domain (1, 13). The first exogenous protein substrates identified for UL97 in vitro were histones (2, 8). More recently, evidence for virus-encoded substrates of UL97 in vitro has emerged with the finding that incubation of lysates of wt HCMV-infected cells with radiolabeled ATP led to the maribavir-sensitive phosphorylation of several viral proteins, including UL44 and UL83. That the effect of maribavir was due to UL97 was confirmed by using lysates from cells infected with a maribavir-resistant UL97 mutant (Sethna et al., submitted). In principle, these proteins might have been phosphorylated not by UL97 directly but rather by a kinase whose activity was controlled by UL97. However, a purified preparation of UL83 was shown to be phosphorylated by recombinant UL97 in a maribavir-sensitive manner (Sethna et al., submitted), and now we have shown here that UL44 is a substrate for purified UL97 in vitro.
To show conclusively that phosphorylation of UL44 was performed by UL97 rather than by a contaminant, we not only modified our purification of the enzyme to remove a contaminant (which was not evident by SDS-PAGE) but also used both a catalytically inactive, mutant form of UL97 (K355Q) and a specific UL97 inhibitor, maribavir. We also found it crucial to remove extraneous serine and threonine residues from UL44 that were left over from fusing it to a histidine tag.
HCMV UL97 is a member of the HvUL protein kinase family (18). It has been reported previously that certain members of this family are capable of phosphorylating exogenous viral proteins. In particular, assays of immunoprecipitates have suggested that the Epstein-Barr virus (EBV) BGLF4 protein can phosphorylate the EBV-encoded analog of HCMV UL44, EA-D (7). Similar studies also have suggested that the varicella-zoster virus ORF 47 protein phosphorylates viral regulatory proteins ORF 62 and ORF 63 (14, 19) and that the herpes simplex virus type 1 (HSV-1) UL13 protein phosphorylates HSV-1 glycoproteins E and I (20). However, in some of these studies, it was possible that phosphorylation occurred on nonviral tag sequences. Perhaps more worrisome, in these studies, in which immunoprecipitates rather than purified proteins were used, it was not clear that the herpesvirus enzyme was the only kinase present. Indeed, mutant forms of BGLF4 and ORF 47, which are akin to UL97 K355Q, retained substantial kinase activity (7, 14), suggesting that the enzyme preparations were indeed contaminated with other kinases. In contrast, the K355Q mutant of HCMV UL97 lacks both autophosphorylation and UL44 phosphorylation activity.
Phosphorylation of viral proteins in infected cells.
Several potential viral substrates for varicella-zoster virus ORF 47 and HSV-1 UL13 have been identified based on their altered phosphorylation in cells infected with null mutant viruses (20-22, 26, 27). However, none of these has been convincingly demonstrated to be a substrate for the relevant viral enzyme in vitro. Here, we have observed altered phosphorylation of UL44 in infected cells following either pharmacological inhibition of UL97 activity by using maribavir or genetic elimination by using a UL97 null mutant. It is possible that the altered phosphorylation observed is due only indirectly to the loss of UL97 activity; however, given that UL44 is a substrate for UL97 in vitro, the simplest interpretation by far is that it is also a substrate of UL97 in infected cells.
The alterations in phosphorylation that we observed were both qualitative and quantitative. The qualitative difference was the loss of a set of phosphorylated species with relatively high pIs (region I and perhaps some of region II in Fig. 6). At first glance, this result is puzzling, because one would expect that the loss of a protein kinase would result in fewer phosphates being added to the protein and thus that removal of kinase activity would result in the loss of the most acidic species (i.e., those with the lowest pIs). However, it appears from our results showing incorporation of 32P into certain UL44 species (region III of the two-dimensional gel in Fig. 6) in the absence of UL97 or its activity, and from other results obtained with lysates of infected cells treated with maribavir (Sethna et al., submitted), that UL44 is phosphorylated via at least one activity other than UL97. It may be that when UL44 is phosphorylated by other kinases, it cannot be phosphorylated by UL97 and vice versa. Indeed, using synthetic peptides derived from a cellular protein that can be phosphorylated by UL97, we have found that phosphates on certain serine residues can prevent phosphorylation by UL97 of nearby residues (M.-C. Baek, P. M. Krosky, A. Pearson, and D. M. Coen, unpublished results). Thus, it may be that UL97 only phosphorylates otherwise unphosphorylated or lightly phosphorylated UL44, which is then not further phosphorylated, resulting in species with relatively high pIs.
There was also a reduction in the amount of radioactivity in region III when UL97 activity was genetically eliminated or pharmacologically inhibited compared to that from untreated wt-infected cells. This may be due to decreased phosphorylation of this species by UL97. Alternatively, it may be due to a reduction in the amount of UL44 protein at late times p.i., which we (P. M. Krosky, M.-C. Baek, and D. M. Coen, unpublished results) and others (33) have observed by Western blot analysis. It is interesting that there is very little, if any, difference in the amounts of UL44 mRNA detected in the presence or absence of UL97 activity (15, 25). Of several possible explanations for this discrepancy, one is that phosphorylation of UL44 affects its stability and thus its accumulation.
What is the role of UL44 phosphorylation by UL97?
UL97 has been reported to have roles in DNA replication, DNA encapsidation, and/or nuclear egress (3, 15, 33). UL44 is best known for its role in viral DNA replication as a subunit of viral DNA polymerase that stimulates long-chain DNA synthesis (10, 24, 28, 32). Thus, it may be that phosphorylation of UL44 by UL97 is important for its function in DNA synthesis. However, although UL97 is very important for production of infectious virus (25), its importance for viral DNA synthesis appears to be variable based on studies in different laboratories (3, 15, 33). UL97 is not required for HCMV oriLyt replication in a cotransfection assay (23). Additionally, phosphorylation by UL97 is not required for the ability of UL44 to interact with and stimulate the catalytic subunit of the polymerase or to bind to DNA, as UL44 expressed in insect cells or in E. coli retains these activities (10, 32; B. Appleton, J. Randell, A. Loregian, J. Hogle, and D. M. Coen, unpublished results). However, phosphorylation of UL44 by UL97 might stimulate these activities. Additionally, given that other kinases can also phosphorylate UL44, it may be that, under certain conditions, cellular protein kinases can fulfill the role of UL97, so that its phosphorylation of UL44 becomes less important.
It is also possible that UL44 has roles other than in viral DNA replication. Its leaky-late kinetics and high level of expression suggest a role after DNA synthesis has progressed (16), which would correspond to when a UL97 null mutant is blocked (15, 33). A report (4) that UL44 can function in transcriptional regulation suggests one function other than DNA replication for this protein. In this regard, HCMV UL44 may be similar to the EBV polymerase accessory protein, EA-D, which has transcriptional regulatory activity (34), although only minor effects on viral gene expression have been detected in UL97 null mutant-infected cells (15, 33). Further studies of UL97 and UL44, aided by maribavir, should shed light on the functions of these proteins and the mechanism of the drug.
Acknowledgments
We thank M. Prichard for graciously providing RCΔ97, P. Ertl and B. Appleton for generous provision of UL44 expression plasmids, H. Zuccola for directions on purifying His6-UL44, W. Britt for kindly supplying monoclonal antibodies 28-19 and 28-21, R. Rando for helpful support of two-dimensional gel electrophoresis studies, S. Gygi for confirmatory mass spectrometric analysis, A. Dickerson for performing amino acid analyses, and S. Ferguson and A. Griffiths for invaluable help with figure preparation.
This work was supported in part by NIH grants AI26077 and AI19838 to D.M.C. P.M.K. was supported in part by NIH training grant T32 AI07245. M.-C.B was supported in part by the Korean Science and Engineering Foundation. Work by W.J.J was supported by NIH grant EY04096 to R. Rando.
REFERENCES
- 1.Baek, M.-C., P. M. Krosky, and D. M. Coen. 2002. The relationship between autophosphorylation and phosphorylation of exogenous substrates by the human cytomegalovirus UL97 protein kinase. J. Virol. 76:11943-11952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek, M.-C., P. M. Krosky, Z. He, and D. M. Coen. 2002. Specific phosphorylation of exogenous protein and peptide substrates by the human cytomegalovirus UL97 protein kinase: importance of the P+5 position. J. Biol. Chem. 277:29593-29599. [DOI] [PubMed] [Google Scholar]
- 3.Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, A. A. Smith, M. G. Davis, C. L. Talarico, R. Ferris, R. E. Dornsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boccuni, M. C., F. Campanini, M. C. Battista, G. Bergamini, P. Dal Monte, A. Ripalti, and M. P. Landini. 1998. Human cytomegalovirus product UL44 downregulates the transactivation of HIV-1 long terminal repeats. AIDS. 12:365-372. [DOI] [PubMed] [Google Scholar]
- 5.Chee, M., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. Hutchinson III, T. Kouzarides, J. A. Martignetti, E. Preddie, S. C. Satchwell, P. Tomlinson, K. M. Weston, and B. G. Barrell. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immun. 154:125-169. [DOI] [PubMed] [Google Scholar]
- 6.Chee, M. S., G. L. Lawrence, and B. G. Barrell. 1989. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J. Gen. Virol. 70:1151-1160. [DOI] [PubMed] [Google Scholar]
- 7.Chen, M.-R., S.-J. Chang, H. Huang, and J.-Y. Chen. 2000. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J. Virol. 74:3093-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coen, D. M., and Z. He. June 1999. UL97 fusion proteins and methods of use. U.S. patent 5,914,244.
- 9.Crossen, R., and S. Gruenwald. 1998. Baculovirus expression vector system manual, 5th ed. Pharmingen, San Diego, Calif.
- 10.Ertl, P. F., and K. L. Powell. 1992. Physical and functional interaction of human cytomegalovirus DNA polymerase and its accessory protein (ICP36) expressed in insect cells. J. Virol. 66:4126-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson, W., T. L. Murphy, and C. Roby. 1981. Cytomegalovirus-infected cells contain a DNA binding protein. Virology 111:251-262. [DOI] [PubMed] [Google Scholar]
- 12.Hanks, S. K., and T. Hunter. 1995. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576-596. [PubMed] [Google Scholar]
- 13.He, Z., Y.-S. He, Y. Kim, L. Chu, C. Ohmstede, K. K. Biron, and D. M. Coen. 1997. The human cytomegalovirus UL97 protein is a protein kinase that phosphorylates on serines and threonines. J. Virol. 71:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenyon, T. K., J. Lynch, J. Hay, W. Ruyechan, and C. Grose. 2001. Varicella-zoster virus ORF47 protein serine kinase: characterization of a cloned, biologically active phosphotransferase and two viral substrates, ORF62 and ORF63. J. Virol. 75:8854-8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krosky, P. M., M. C. Baek, and D. M. Coen. 2003. Human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leach, F. S., and E. S. Mocarski. 1989. Regulation of cytomegalovirus late-gene expression: differential use of three start sites in the transcriptional activation of ICP36 gene expression. J. Virol. 63:1783-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Littler, E., A. D. Stuart, and M. S. Chee. 1992. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature 358:160-162. [DOI] [PubMed] [Google Scholar]
- 18.McGeoch, D. J., L. J. Coulter, and H. W. M. Moss. 1995. UL protein kinases (herpesviruses), p. 391-393. In D. G. Hardie and S. Hanks (ed.), The protein kinase facts book, vol. 1. Academic Press, London, United Kingdom.
- 19.Ng, T. I., L. Keenan, P. R. Kinchington, and C. Grose. 1994. Phosphorylation of varicella-zoster virus open reading frame (ORF) 62 regulatory product by viral ORF 47-associated protein kinase. J. Virol. 68:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng, T. I., W. O. Ogle, and B. Roizman. 1998. UL13 protein kinase of herpes simplex virus 1 complexes with glycoprotein E and mediates the phosphorylation of the viral Fc receptor: glycoproteins E and I. Virology 241:37-48. [DOI] [PubMed] [Google Scholar]
- 21.Ng, T. I., C. Talarico, T. C. Burnette, K. Biron, and B. Roizman. 1996. Partial substitution of the functions of the herpes simplex virus UL13 gene by the human cytomegalovirus UL97 gene. Virology 225:347-358. [DOI] [PubMed] [Google Scholar]
- 22.Ogle, W. O., T. I. Ng, K. L. Carter, and B. Roizman. 1997. The UL13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology 235:406-413. [DOI] [PubMed] [Google Scholar]
- 23.Pari, G. S., and D. G. Anders. 1993. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J. Virol. 67:6979-6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pari, G. S., M. A. Kacica, and D. G. Anders. 1993. Open reading frames UL44, IRS1/TRS1, and UL36-38 are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA synthesis. J. Virol. 67:2575-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prichard, M. N., N. Gao, S. Jairath, G. Mulamba, P. Krosky, D. M. Coen, B. O. Parker, and G. S. Pari. 1999. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J. Virol. 73:5663-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purves, F. C., W. O. Ogle, and B. Roizman. 1993. Processing of the herpes simplex virus regulatory protein α22 mediated by the UL13 protein kinase determines the accumulation of a subset of α and γ mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. USA 90:6701-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy, S. M., E. Cox, I. Iofin, W. Soong, and J. I. Cohen. 1998. Varicella-zoster virus (VZV) ORF32 encodes a phosphoprotein that is posttranslationally modified by the VZV ORF47 protein kinase. J. Virol. 72:8083-8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ripalti, A., M. C. Boccuni, F. Campanini, and M. P. Landini. 1995. Cytomegalovirus-mediated induction of antisense mRNA expression to UL44 inhibits virus replication in an astrocytoma cell line: identification of an essential gene. J. Virol. 69:2047-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan, V., C. L. Talarico, S. C. Stanat, M. Davis, D. M. Coen, and K. K. Biron. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 358:162-164. [DOI] [PubMed] [Google Scholar]
- 30.Talarico, C. L., T. C. Burnette, W. H. Miller, S. L. Smith, M. G. Davis, S. C. Stanat, T. I. Ng, Z. He, D. M. Coen, B. Roizman, and K. K. Biron. 1999. Acyclovir is phosphorylated by the human cytomegalovirus UL97 protein. Antimicrob. Agents Chemother. 43:1941-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turk, S. R., C. Shipman, Jr., R. Nassiri, G. Genzlinger, S. H. Krawczyk, L. B. Townsend, and J. C. Drach. 1987. Pyrrolo[2,3-d]pyrimidine nucleosides as inhibitors of human cytomegalovirus. Antimicrob. Agents Chemother. 31:544-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiland, K. L., N. L. Oien, F. Homa, and M. W. Wathen. 1994. Functional analysis of human cytomegalovirus polymerase accessory protein. Virus Res. 34:191-206. [DOI] [PubMed] [Google Scholar]
- 33.Wolf, D. G., C. T. Courcelle, M. N. Prichard, and E. S. Mocarski. 2001. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc. Natl. Acad. Sci. USA 98:1895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang, Q., Y. Hong, D. Dorsky, E. Holley-Guthrie, S. Zalani, N. A. Elshiekh, A. Kiehl, T. Le, and S. Kenney. 1996. Functional and physical interactions between the Epstein-Barr virus (EBV) proteins BZLF1 and BMRF1: effects on EBV transcription and lytic replication. J. Virol. 70:5131-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuccola, H. Z., D. J. Filman, D. M. Coen, and J. M. Hogle. 2000. The crystal structure of an unusual processivity factor, herpes simplex virus UL42, bound to the C terminus of its cognate polymerase. Mol. Cell 5:267-278. [DOI] [PubMed] [Google Scholar]