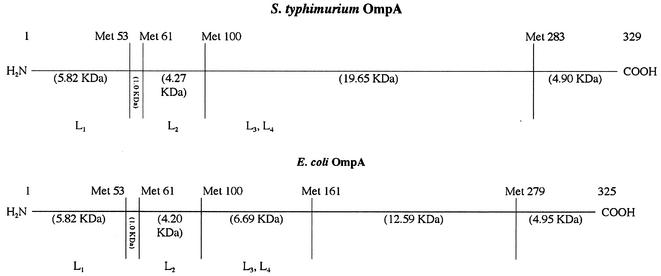
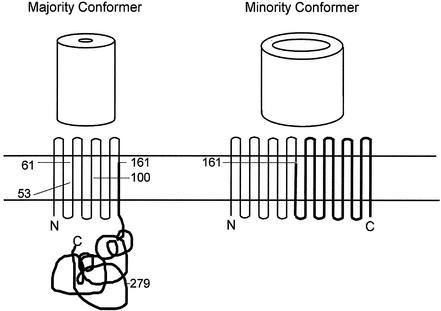
FIG. 5.
Identification of OmpA domain bound by anti-OmpA MAbs. The Met positions and the corresponding molecular weights of the expected CNBr-generated fragments are shown for mature OmpA of serovar Typhimurium and E. coli in the top panel. Surface-exposed loops L1 (amino acids 17 to 33), L2 (amino acids 61 to 71), L3 (amino acids 107 to 114), and L4 (amino acids 144 to 160) that are localized in the N-terminal domain are also shown. Transmembrane topology for the majority and minority conformers of OmpA is shown at the bottom, with the location of the methionine residues (in the E. coli protein) indicated for the majority conformer. The C-terminal fragment, bearing the major epitope, is shown as thick lines.