Figure 3.
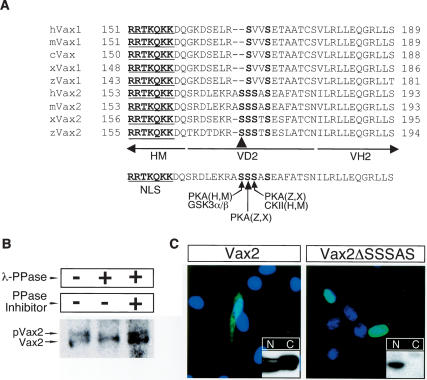
The SRS in VD2 is essential for the cytoplasmic localization of Vax2. (A) Amino acid sequence alignment of the cytoplasmic retention domain (VD2) of various Vax1 and Vax2 proteins. (H) human; (M) mouse; (X) Xenopus; (Z) zebrafish. The Vax2 VD2 carries an SRS that contains potential target sites for phosphorylation by PKA and CKII. The arrowhead marks Ser 170 in mouse Vax2. (B) Vax2 is a phospho-protein in vivo. Vax2 protein was isolated by immunoprecipitation from Schwann cells, followed by treatment with λ protein phosphatase (λ-PPase; New England Bio-Labs; 400 U) in the presence or absence of the phosphatase inhibitor cocktail 2 (PPase Inhibitor; Sigma), and was analyzed by 10% SDS-PAGE and Western blotting with anti-Vax2 antibody. (C) Essential role of SRS in the cytoplasmic retention of Vax2. Myc-tagged wild-type mouse Vax2 or Vax2ΔSSSAS, a deletion mutant lacking amino acids 170–174 of mVax2, was expressed in Schwann cells, and its subcellular localization was monitored by immunostaining with anti-Myc antibody (green) and DAPI (blue). Boxed insets are immuno-blots with anti-Myc antibody detecting Myc-tagged Vax2 proteins in the cytoplasmic (C) or in the nuclear (N) fractions of the transfected cells, as for Figure 2B.