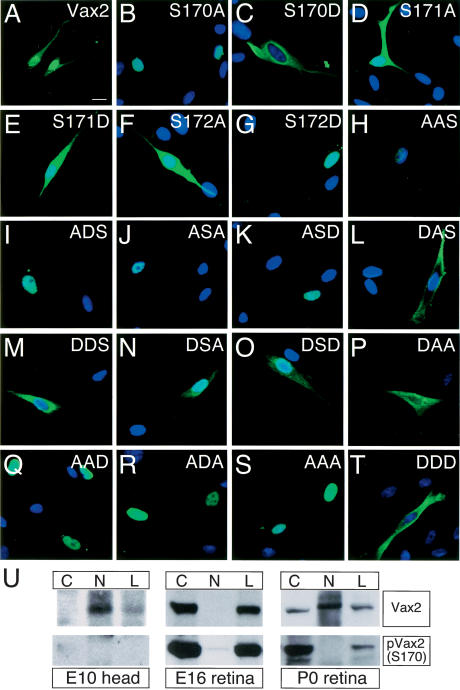
Figure 4.
Phosphorylation of S170 is required for the cytoplasmic localization of Vax2. Schwann cells were transfected with a variety of Myc-Vax2 mutant cDNAs that encode Vax2 proteins with single, double, and triple mutations of Ser 170, Ser 172, and Ser 174 to either alanine or aspartic acid. Subcellular localization of the mutant proteins was monitored by immunostaining with anti-Myc antibody (green) and DAPI (blue). S170A, for example, is a Vax2 protein in which Ser 170 is mutated to alanine (B) and DSD is a Vax2 protein in which both Ser 170 and Ser 174 are mutated to aspartic acid (O). See text for details. (U) S170 phosphorylation of Vax2 marks cytoplasmic localization in vivo. Cell lysates (L) from embryonic heads (E10.5) or retina (E16 and P0) were separated into cytoplasmic (C) and nuclear (N) fractions by low-speed centrifugation, and then analyzed for the presence of Vax2 and S170-phosphoylated Vax2 [pVax2(S170)] by Western blotting with the indicated antibodies.