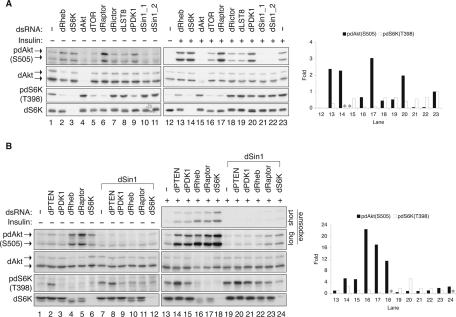
FIGURE 1.
dSin1 is essential for dAkt phosphorylation in Drosophila S2 cells. (A) dSin1 knockdown inhibits both basal and insulin-stimulated dAkt phosphorylation but not dS6K phosphorylation. dsRNA was used to target an individual dTOR pathway component as indicated. “dSin1_1” and “dSin1_2” denote two nonoverlapping dsRNAs targeting different regions of the dSin1 mRNA. Insulin stimulation (100 nM, 15 min) is indicated. Protein levels and phosphorylation were determined by Western blotting with indicated antibodies. The dAkt protein levels were detected by mammalian Akt antibody. The two dAkt isoforms generated by alternative splicing are indicated by the arrows. The column chart shows the quantification results (ImageJ) of the dAkt and dS6K phosphorylation under insulin stimulation condition. The lane numbers in the chart correspond to those labeled at the bottom of the Western blot panels. (*) Knockdown of either dAkt or dS6K that led to undetectable protein levels for quantification. The phosphorylation under basal condition was too low to be accurately quantified. (B) dSin1 knockdown blocks the effects caused by knockdown of other TOR pathway components on dAkt but not on dS6K phosphorylation. RNAi experiments, insulin stimulation, Western blotting, and quantification were performed the same as described in A. Where indicated, dSin1 dsRNA was used in combination and added to the cells at the same time with the other dsRNAs.