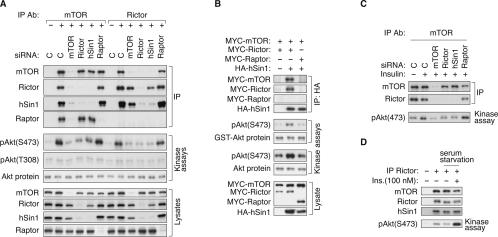
FIGURE 4.
hSin1 is important for TORC2 kinase activity. (A) hSin1 is indispensable for TORC2 kinase activity in vitro. HeLa cells were transfected with each indicated siRNA and lysed in CHAPS buffer. “C” denotes control siRNA targeting GFP. mTOR and Rictor IPs were performed as indicated. Dephosphorylated Akt (Upstate Biotechnology, from insect cells) was used as a substrate for the in vitro TORC2 kinase assay. Phosphorylation of Akt was first determined by the phospho-Akt(S473) antibody, and then reprobed by the phospho-Akt(T308) antibody after low pH stripping. Note that the pAkt(T308) blot had a very long exposure time and only shows the background signal. A small fraction of the kinase reactions was blotted with Akt antibody for protein levels. (B) hSin1 directly precipitates active TORC2 kinase. HEK293 cells were transfected with indicated plasmids, lysed in CHAPS buffer, and immunoprecipitated with HA antibody. Purified GST-Akt (from HEK293 cells) and the dephosphorylated Akt (Upstate Biotechnology) were used as substrates for the kinase assays. The phosphorylation of the Akt substrates was determined by Western blotting with phospho-specific antibody as indicated. Protein levels were determined by HA, MYC, GST, and Akt antibodies. (C) hSin1 is required for insulin-stimulated TORC2 activity. HeLa cells were transfected with various siRNAs as indicated. Cells were stimulated with insulin (100 nM, 15 min) and lysed in CHAPS buffer. In vitro kinase assay of immunoprecipitated mTOR was performed using dephosphorylated Akt as a substrate. (D) Insulin stimulation does not alter TORC2 integrity. HeLa cells were serum-starved overnight before being treated with insulin (100 nM, 15 min). Cells were harvested with CHAPS buffer and the lysates were subjected to Rictor immunoprecipitation. TORC2 kinase activity was assayed using dephosphorylated Akt as a substrate.