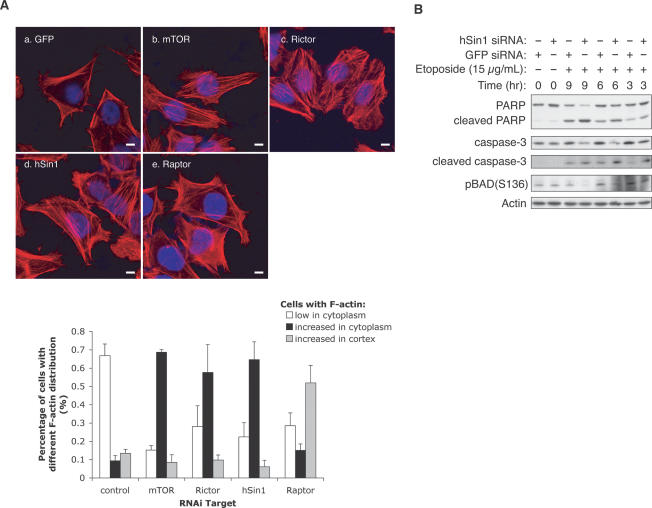
FIGURE 5.
hSin1 plays a role in actin organization and cell survival. (A) hSin1 RNAi displays similar actin fiber organization as Rictor RNAi. HeLa cells were transfected with siRNA against GFP control (a), mTOR (b), Rictor (c), hSin1 (d), and Raptor (e). Thirty-six hours post-transfection, cells were serum-starved for 12 h, followed by insulin stimulation (200 nM, 30 min) before fixation. F-actin (red) and nucleus (blue) were visualized under confocal microscope (bars, 10 μm). The column chart shows the quantification results of the change on cellular F-actin organization. For each knockdown, three sample groups were counted. Cells were divided into three categories based on F-actin staining: those with few stress fibers (<20% of the cell area, open bars), those with intense cytoplasmic stress fibers (filled bars), and those with strong Factin in the cortex (hatched bars). Quantification was determined by visual counting of confocal images. (B) hSin1 knockdown sensitizes cells to apoptosis. HeLa cells were transfected with either hSin1 or GFP (control) siRNA. Cells were treated with 15 μg/mL etoposide at 9, 6, and 3 h prior to harvesting; lysed in NP40 buffer; and analyzed by Western blotting. PARP antibody was used to detect both full-length and cleaved PARP. Caspase-3 antibody and caspase-3 (Asp175) antibody were used to detect procaspase-3 (full length) and cleaved caspase-3, respectively. Phosphorylation of BAD was determined by phospho-specific antibody as indicated. Note that the hSin1 RNAi treatment at T = 0 is shorter than the other experiments because the cells were treated with etopside for additional hours. This may explain a lack of effect of hSin1 RNAi on BAD phosphorylation. Additionally, BAD can be phosphorylated by other kinases besides Akt.