Abstract
The mesolimbic dopamine system projects to a large number of forebrain areas and plays an important role in the regulation of locomotor activity, cognition and reward. We previously found evidence for a functional interaction between the mesolimbic dopamine system and circulating vasopressin and the present study was performed to test the hypothesis that mesolimbic dopamine stimulation modulates the cardiovascular effects of vasopressin.
Sprague-Dawley rats were stereotaxically implanted with a guide cannula into the region of origin of the mesolimbic system, the ventral tegmental area, and instrumented with catheters into the abdominal aorta and jugular vein. One week later, separate groups of conscious rats were injected intravenously with 1, 3 or 10 ng kg−1 of arginine-vasopressin or other vasopressor drugs before and after intra-ventral tegmental area injection of 10 nmol of neurotensin.
Intra-ventral tegmental area injections of neurotensin had no significant effect on mean arterial pressure and heart rate but significantly potentiated the pressor response to intravenous administration of vasopressin when compared to saline-injections. However, the vasopressin-induced bradycardia was unaffected. Intravenous pretreatment with raclopride blocked the ability of neurotensin, injected into the ventral tegmental area, to potentiate the vasopressin-induced pressor response. Intra ventral tegmental area injections of neurotensin had no effect on the pressor response and bradycardia induced by intravenous angiotensin II or methoxamine.
In conclusion, these results suggest that the mesolimbic dopamine system, in addition to its well-known role in the regulation of behaviour, modulates cardiovascular control by potentiating the effects of vasopressin on mean arterial pressure.
Keywords: Dopamine, ventral tegmental area, mesolimbic, vasopressin, mean arterial pressure, angiotensin, methoxamine, baroreflex, brain
Introduction
The ventral tegmental area in the ventral midbrain contains a group of tyrosine hydroxylase immunoreactive cells that are commonly known as the A10 dopaminergic cell group. These cells send a large number of projections to several forebrain areas, most notably the nucleus accumbens, frontal cortex, olfactory tubercle, islands of Calleja, and amygdala (Lindvall & Björklund, 1974; Oades & Halliday, 1987; Swanson, 1982). Together, these projections form the mesolimbic/mesocortical dopamine system in the brain. This system has been implicated in the regulation of locomotor activity, reward mechanisms, cognition and stress responses (see Dunnett & Robbins, 1992; Kalivas et al., 1993; Le Moal & Simon, 1991). We recently investigated the role of the ventral tegmental area in cardiovascular regulation (see Van den Buuse, 1998). We stimulated ventral tegmental area cells by micro-injecting the metabolically stable substance P analogue DiMe-C7 (Eison et al., 1982b) into this region in conscious rats. This treatment was shown by others to cause an increase in dopamine turnover in forebrain regions (Elliott et al., 1986) and to induce behavioural effects consistent with mesolimbic dopaminergic stimulation (Eison et al., 1982a). We found a prolonged and dose-dependent increase in mean arterial pressure after infusion of DiMe-C7 into the ventral tegmental area (Cornish & Van den Buuse, 1994; 1995). The pressor response could be prevented by pretreatment of the rats with the dopamine receptor antagonists haloperidol (Cornish & Van den Buuse, 1994), raclopride and SCH23390 (Cornish & Van den Buuse, 1995), suggesting that central dopamine release and dopamine D1 and D2 receptor activation was involved in the effects on mean arterial pressure. Intravenous pretreatment with a vasopressin V1 receptor antagonist also prevented the pressor response to stimulation of the ventral tegmental area with DiMe-C7 (Cornish & Van den Buuse, 1995), suggesting it was mediated by the release of vasopressin into the circulation. Indeed, ventral tegmental area stimulation with DiMe-C7 caused a modest, but significant increase in plasma vasopressin levels (Cornish et al., 1997) and marked cellular activity in the supraoptic nucleus of the hypothalamus, a major source of pituitary vasopressin (Cornish & Van den Buuse, 1996). Surprisingly, however, unlike the pressor response, the stimulation of vasopressin release was not affected by pretreatment with raclopride (Cornish et al., 1997). We then postulated that stimulation of the ventral tegmental area by DiMe-C7 has two effects: firstly vasopressin release is stimulated by a non-dopaminergic mechanism which by itself is not sufficient to induce a pressor response; secondly mesolimbic dopaminergic stimulation somehow modulates cardiovascular buffering mechanisms to enable the increase in vasopressin levels to induce a pressor response (Cornish et al., 1997; see also Van den Buuse, 1998). The effects of vasopressin on mean arterial pressure are normally buffered by baroreflex mechanisms, possibly to inhibit its pressor action in favour of its diuretic action (see Cowley & Liard, 1988; Cowley et al., 1983; Schmid et al., 1984; Webb et al., 1986). Mesolimbic dopaminergic stimulation may alleviate some of this buffering which could be important in situations of behavioural activation, stress or high salt intake (see Van den Buuse, 1998). Consistent with this hypothesis, we found that micro-injection of DiMe-C7 into the ventral tegmental area caused a significant reduction in baroreceptor-heart reflex sensitivity, an effect not seen after raclopride pretreatment (see Van den Buuse, 1998; Van den Buuse et al., 1998).
The proposed effect of stimulation of the ventral tegmental area on blood pressure regulation (see Van den Buuse, 1998) predicts that a ‘pure' dopaminergic stimulation of this region would not alter resting mean arterial pressure, but would alter the cardiovascular responses to subsequent pressor stimuli, particularly exogenously administered vasopressin. The present study was performed to test this hypothesis. We used stimulation of the ventral tegmental area by micro-injection of neurotensin which has been shown to potently activate dopaminergic cells in this region (see Kalivas, 1993; Kalivas et al., 1983). We firstly compared the effect of neurotensin on resting mean arterial pressure and vasopressin release with that of DiMe-C7. We then administered vasopressin and other pressor stimuli before and after neurotensin stimulation of the ventral tegmental area with neurotensin.
Methods
Rats, surgery
A total of 90 male Sprague-Dawley rats of 250–300 g body weight were instrumented as described in detail before (Cornish & Van den Buuse, 1995; Van den Buuse et al., 1996). Briefly, the animals were anaesthetized with pentobarbitone (60 mg kg−1 intraperitoneally) and mounted in a Kopf stereotaxic apparatus. A stainless steel guide cannula (C313-G, Plastic Products, Roanoke, VA, U.S.A.) was implanted unilaterally at an angle of 12° and stereotaxic coordinates 5.3 mm posterior, 2.5 mm lateral, and 8.5 mm ventral of the bregma. The cannula was kept in place with miniature screws and dental cement and closed with a dummy cannula (C313-DC). The rats were administered 0.15 mg kg−1 of buprenorphine (Temgesic) and allowed to recover for 5–7 days. For measurement of mean arterial pressure and intravenous administration of compounds, the rats were instrumented with a vinyl/teflon catheter into the abdominal aorta and a vinyl catheter into one jugular vein, respectively. Anaesthesia was induced by intraperitoneal administration of a mixture of pentobarbital (30 mg kg−1), methohexitone (50 mg kg−1) and atropine sulphate. After surgery, all rats were again administered Temgesic before allowed to recover for another 5–7 days.
All surgical techniques, treatments and experimental protocols were performed in accordance with the Australian Code of Practise for the Care and Use of Animals for Scientific Purposes (1990) set out by the National Health and Medical Research Council of Australia.
Experimental protocol
On the day of the experiment, the rats were weighed and allowed at least 1 h to acclimatize in the experiment room. The rats were freely moving in their home cages and had access to food pellets. Water bottles were removed throughout the experiment. Mean arterial pressure (MAP) was measured with Gould P23ID or P23XL transducers and recorded on an eight channel Neotrace recorder (Neomedix Systems, Australia). Heart rate was obtained from the pressure pulse by Baker Medical Research Institute tachographs. Mean arterial pressure and heart rate were averaged and collected at 2 s intervals with a Labview-based data acquisition program developed at the Baker Medical Research Institute.
In the first experiments, the rats were micro-injected into the ventral tegmental area with either saline (n=10), 10 nmol of DiMe-C7 (n=10) or 10 nmol of neurotensin (n=10) in saline. Mean arterial pressure and heart rate were recorded during 10 min before and 60 min after central micro-injections. A second group of rats was similarly treated but decapitated 10 min after micro-injection of saline (n=7), DiMe-C7 (n=10) or neurotensin (n=6). Trunk blood was obtained from these rats for the analysis of plasma vasopressin levels. This time-point was chosen because previous experiments had shown that the pressor response to micro-injections of DiMe-C7 into the ventral tegmental area was maximal (Cornish & Van den Buuse, 1995; Cornish et al., 1997).
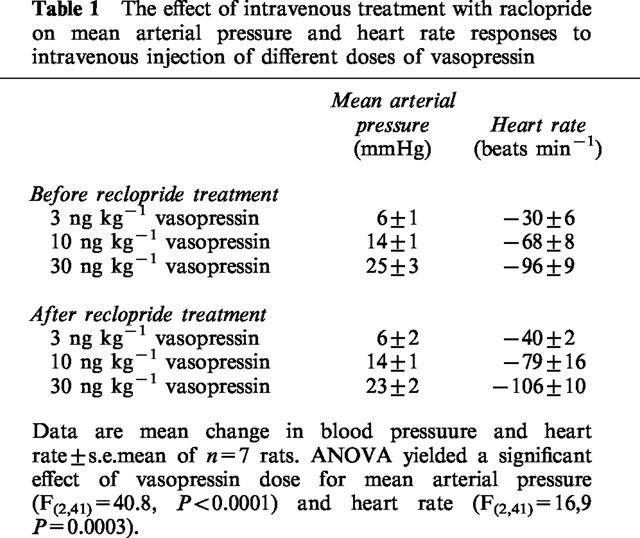
In the second series of experiments, arginine-vasopressin (1, 3, 10 ng kg−1), angiotensin II (3, 10, 30 ng kg−1) or methoxamine (20, 40, 60 μg kg−1) were injected intravenously at 5 min intervals. Neurotensin (n=10, 7 and 6, respectively) was micro-injected into the ventral tegmental area 10 min after the highest dose and, commencing 5 min later, the intravenous dose response curve was repeated. In controls, saline was injected into the ventral tegmental area (n=10, 7 and 4, respectively). Intravenous injections were thus done at 20, 15 and 10 min before and at 5, 10 and 15 min after central treatment. This protocol was chosen on the basis of the time course of the pressor effect of DiMe-C7 which is maximal between 5 and 15 min after injection into the ventral tegmental area. Responses to vasopressin were also measured before and after injection of neurotensin (n=9) or saline (n=7) into the ventral tegmental area following pretreatment with 0.5 mg kg−1 of the dopamine D2 receptor antagonist, raclopride (Hall et al., 1988). A preliminary experiment was also conducted to establish whether raclopride treatment per se caused changes in the effect of vasopressin injections on mean arterial pressure or heart rate (n=7, Table 1). Doses of the vasoconstrictor agents were chosen from preliminary experiments according to their ability to cause an increase in mean arterial pressure comparable to that after central micro-injection of DiMe-C7 (Cornish & Van den Buuse, 1995; Cornish et al., 1997). The dose of raclopride was chosen on the basis of literature data and because it was previously shown to block the pressor responses induced by the dopamine D2 receptor agonist quinpirole (Van den Buuse, 1992).
Table 1.
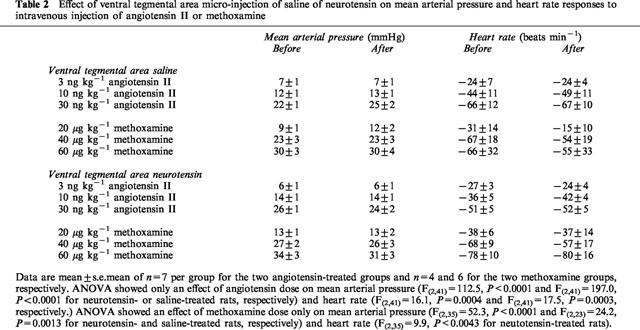
Effect of ventral tegmental area micro-injection of saline of neurotensin on mean arterial pressure and heart rate responses to intravenous injection of angiotensin II or methoxamine
Central unilateral micro-injections were 1.0 μl infused over 1 min with a Hamilton 10 μl micro-syringe and a Harvard Apparatus syringe infusion pump (Model 22, MA, U.S.A.). The injection system consisted of a stainless steel injection needle (Plastic Products Company, VA, U.S.A.) connected to 50–60 cm of fine tubing (SV31, Dural Plastics, NSW, Australia). The central infusion commenced approximately 1 min after insertion of the injection needle into the guide cannula. The needle was removed approximately 2 min after drug infusion to allow complete diffusion. Rats were not restrained during the injection procedure. Where individual animals were used more than once, treatments were given in random order on separate occasions, with at least 48 h between the experiments.
At the completion of experiments the animals were killed by an intravenous overdose of pentobarbitone sodium (350 mg ml−1, Euthatal). Brains were dissected out and placed in a solution of 4% formaldehyde for at least 24 h to fix brain tissue for histological verification of the central injection site. Only rats with correct placement of the central guide cannula, into or immediately above the ventral tegmental area, were used (Cornish & Van den Buuse, 1995).
Vasopressin radio-immunoassay
Plasma levels of vasopressin were analysed as previously described (Woods & Johnston, 1983). Briefly, duplicate 1 ml basal and spiked samples of each rat were extracted with acetone and petroleum ether. Reconstituted, extracted plasma samples were assayed using an antibody raised in rabbits (1 : 30,000) by Dr R. Woods, Baker Medical Research Institute, and [125I]-vasopressin synthesized by M. Fullerton, Baker Medical Research Institute. Synthetic arginine-vasopressin was used as a standard. Assay sensitivity was 1.0 pg ml−1 of plasma.
Drugs and solutions
All drug concentrations are expressed as weight of the salt per volume of vehicle. Rats were anaesthetized with a mixture of pentobarbitone and methohexitone with atropine sulphate. The mixture was made up of 5 mls of Nembutal (pentobarbitone sodium, 60 mg ml−1, Boehringer Ingelheim, Animal Health Division, Atarmon, New South Wales, Australia), 40 mls of Brietal (methohexitone sodium, 500 mg, Eli Lilly Australia, West Ryde, New South Wales, Australia), and 5 mls of atropine sulphate (0.6 mg ml−1, Aphex Laboratories, St. Marys, New South Wales, Australia). The final doses were 30 mg kg−1, 50 mg kg−1 and 0.3 mg kg−1, respectively. Pain relief was provided by administration of Temgesic (0.15 mg ml−1 of buprenorphine, Rickett and Coleman Pharmaceuticals, New South Wales, Australia).
All peptides in this study were obtained from Auspep, Melbourne, Australia, except arginine vasopressin used in radio-immunoassays which was obtained from Peninsula Laboratories, CA, U.S.A. Peptides included DiMe-C7 (pGlu-Gln - Phe - Me - Phe - Sar -Leu -Met-NH2, [pGu5,MePhe8,Sar9]-Substance P5-11), neurotensin (pGlu-Leu-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu-OH), arginine-vasopressin (H-Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly-NH2), and angiotensin II (H-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-OH). All peptides were dissolved in a minimal amount of glacial acetic acid and diluted with physiological saline to the required concentration. Methoxamine HCl was obtained from Sigma, St. Louis, MO, U.S.A., and was dissolved in at 20, 40 or 60 μg ml−1 in physiological saline. Raclopride tartrate was a gift from Dr David Jackson, Astra, Södertalje, Sweden, and was dissolved at 0.5 mg ml−1 in physiological saline.
Data analysis
Data are expressed as mean change in mean arterial pressure or heart rate±standard error of the mean (s.e.mean). In the case of vasoconstrictor dose-response experiments, maximal increases in mean arterial pressure were taken and the difference with baseline mean arterial pressure immediately before the intravenous injection calculated. Heart rate exchanges were calculated at the same time-point as the pressor responses.
The effect of central injection of saline, DiMe-C7 or neurotensin on mean arterial pressure and heart rate was analysed with a two-way analysis of variance (ANOVA) for repeated measures using treatment as the between-group factor and time after injection as the within subject factor. Between-group differences were further analysed with a Bonferroni-corrected t-test.
The dose-response curves of vasopressin, angiotensin II or methoxamine on mean arterial pressure and heart rate were analysed with a two-way ANOVA for repeated measures using treatment (data before vs after central micro-injection) and intravenous dose as within-subject factors. Between-group comparisons were again done with a Bonferroni-corrected t-test. All statistical analysis was carried out using Sigmastat, version 1.0 (Jandel Scientific). A P<0.05 was taken as indicating a statistically significant difference between groups.
Results
Effect of ventral tegmental area injections on mean arterial pressure and heart rate
As shown in Figure 1, micro-injection of 10 nmol of neurotensin into the ventral tegmental area caused little effect on mean arterial pressure (average response over 60 min 4±1 mm Hg). By contrast, the micro-injection of 10 nmol of DiMe-C7 produced a prolonged and highly significant increase in mean arterial pressure (average response 13±1 mmHg) which peaked at 10 min after treatment. Injection of saline did not cause changes in mean arterial pressure (average response −2±1 mmHg) (Figure 1).
Figure 1.
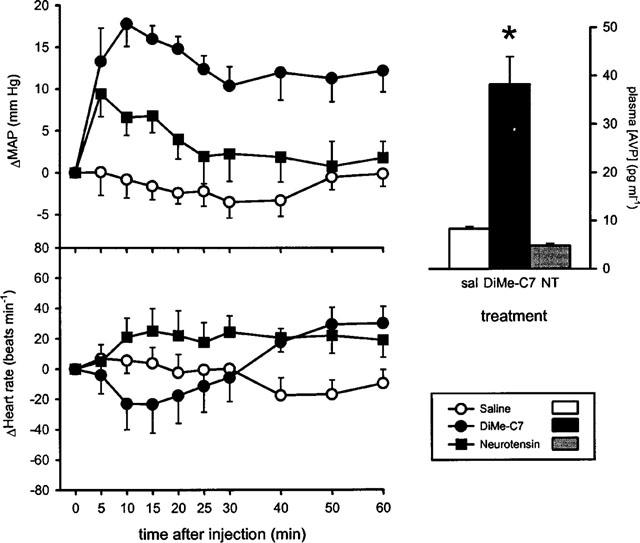
Left panel: The effect of micro-injection of saline or 10 nmol of the substance P analogue DiMe-C7 or neurotensin into the ventral tegmental area on mean arterial pressure (MAP, top) and heart rate (bottom) of conscious rats (n=10 rats per group). ANOVA showed that there was an overall significant effect of treatment (F(2,269)=17.l, P<0.0001) and of time after injection (F(8,269=3.6, P=0.0005). Between-group analysis showed that the changes in mean arterial pressure after treatment with DiMe-C7 were significantly different from those after saline or neurotensin treatment which were not different from each other. Further between-group analysis showed that the change in mean arterial pressure after treatment with DiMe-C7 was significantly greater than that after treatment with saline at 10, 15, 20, 25, 30, and 40 min after injection (P<0.05, Bonferroni-corrected t-test). At none of the time-points was the small increase in mean arterial pressure in rats treated with neurotensin significantly different from that in rats treated with saline. No significant differences in heart rate were observed. Right panel: The effect of micro-injection of saline (n=7), DiMe-C7 (n=10) or neurotensin (n=6) into the ventral tegmental area on plasma vasopressin concentration at 10 min after stimulation. Data are mean±s.e.mean. *P<0.05 for difference with saline-injected controls.
Micro-injection of neither saline, DiMe-C7 or neurotensin into the ventral tegmental area caused consistent changes in heart rate (average changes over 60 min −3±3, −1±5, and 20±4 beats min−1, respectively) and there were no significant differences between any of the groups (Figure 1).
In a separate group of rats, plasma vasopressin levels were measured at 10 min after injection of either saline, DiMe-C7 or neurotensin into the ventral tegmental area (Figure 1). Vasopressin levels were significantly higher after treatment with DiMe-C7, but not neurotensin, when compared to saline-treated controls. The effect of DiMe-C7 on mean arterial pressure, heart rate and plasma vasopressin levels was in agreement with previous studies (Cornish & Van den Buuse, 1995; Cornish et al., 1997).
Rats that were injected into the ventral tegmental area with saline showed no noticeable changes in behaviour and would often sleep through most of the experiment. After DiMe-C7 treatment, the rats showed quiet wakefulness or mild locomotor activation and typically showed occasional ‘wet-dog shaking' behaviour. After neurotensin treatment, quiet wakefulness and mild locomotor activation was observed.
Effect of stimulation of the ventral tegmental area on the action of vasopressin
Intravenous injection of 1, 3 or 10 ng kg−1 of vasopressin caused a dose-dependent increase in mean arterial pressure which lasted 3–5 min (Figure 2). Repeating the dose-response curve in rats which had been micro-injected with 10 nmol of neurotensin into the ventral tegmental area produced significantly greater responses (Figures 2 and 3). The average increase in the effect of vasopressin on mean arterial pressure was 5±1 (56% increase), 6±1 (37%) and 4±1 mmHg (20%), for the 1, 3, and 10 ng kg−1 doses, respectively. By contrast, there was no significant difference in the dose-response curve to vasopressin before and after injection of saline into the ventral tegmental area (Figures 2 and 3).
Figure 2.
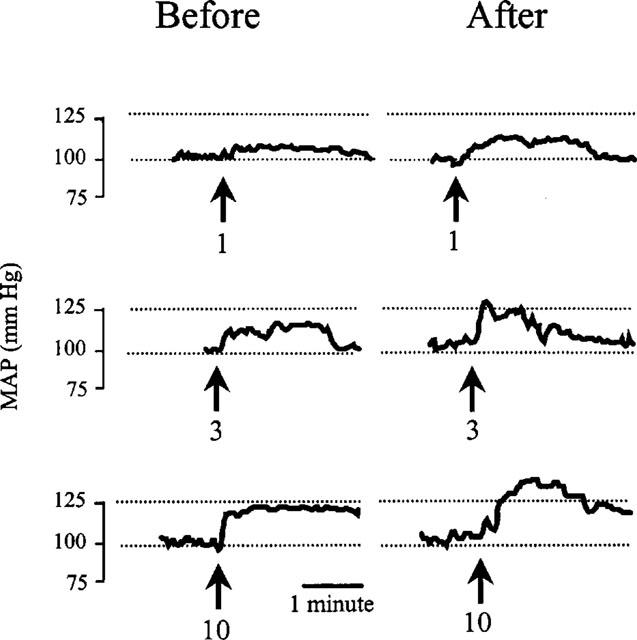
Typical traces of blood pressure recordings showing the mean arterial pressure (MAP) responses to intravenous injections of 1, 3, or 10 ng kg−1 of arginine-vasopressin before (left panels) and after (right panels) micro-injection of 10 nmol of neurotensin into the ventral tegmental area of conscious rats. Arrows indicate the moment of vasopressin injections. Numbers wth the arrows refer to the vasopressin doses in ng kg−1.
Figure 3.
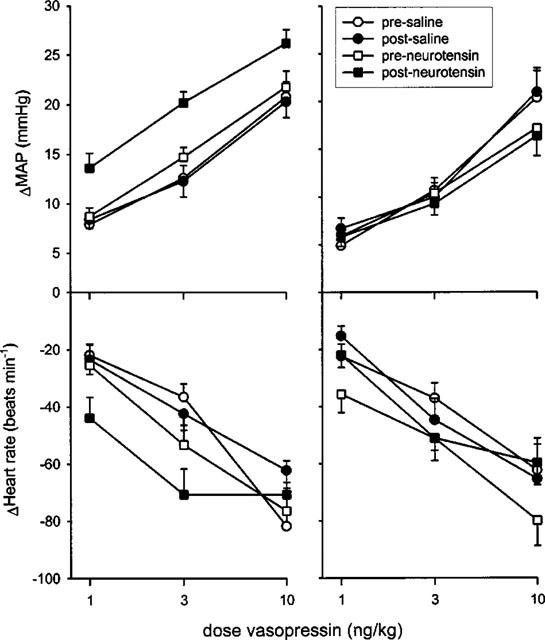
Maximal changes in mean arterial pressure (MAP, mmHg, top panels) and heart rate (beats min−1, bottom panels) following intravenous injection of arginine-vasopressin before and after micro-injection of neurotensin or saline into the ventral tegmental area of conscious rats. The rats were either not pretreated (left panels) or pretreated with raclopride (right panels). For pressor responses in controls, ANOVA yielded a significant effect of neurotensin treatment (F(1,59)=18.0, P<0.0022) and of vasopressin dose (F(2,59)=66.3, P<0.0001). After injection of saline into the ventral tegmental area, ANOVA yielded a significant effect of vasopressin dose only (F(2,59)=65.6, P<0.0001). For heart rate responses, ANOVA yielded significant effects of vasopression dose only (F(2,59)=11.0, P=0.0008 and F(2,59)=23.1, P<0.0001 after neurotensin- or saline injection, respectively). Similarly, for pressor responses in raclopride-pretreated rats, ANOVA yielded only a significant effect of vasopressin dose (F(2,53)=83.1, P<0.0001 and F(2,41)=48.6, P<0.0001 after neurotensin- or saline injection, respectively). For heart rate responses in raclopride-pretreated rats again there was only a significant effect of vasopressin dose (F2,53)=25.9, P<0.0001, and F(2,41)=17.0, P=0.0003, respectively). Data are mean change in mean arterial pressure (mmHg) or heart rate (beats min−1) ±s.e.mean of n=10 for saline- and neurotensin-treated controls and n=7 and 9 for the saline and neurotensin-treated raclopride groups, respectively
Intravenous injection of 1, 3 or 10 ng kg−1 of vasopressin caused a dose-dependent decrease in heart rate. The bradycardia response to vasopressin treatment was not significantly different before and after micro-injection of neurotensin or saline into the ventral tegmental area (Figure 3).
Intravenous treatment of rats with 0.5 mg kg−1 of the dopamine D2 receptor antagonist raclopride did not cause any changes in resting mean arterial pressure and heart rate (not shown). In addition, treatment with raclopride per se did not influence the effect of intravenous administration of vasopressin on mean arterial pressure and heart rate (Table 1). In rats which had been pretreated with raclopride, intravenous injections of 1, 3 or 10 ng kg−1 of vasopressin again caused dose-dependent pressor responses and bradycardia. In contrast to what was found in controls, however (see above), after raclopride pretreatment neurotensin injections into the ventral tegmental area failed to enhance the pressor action of vasopressin (Figure 3). In raclopride-pretreated rats, saline injection into the ventral tegmental area again produced no significant changes in the dose-response curve of vasopressin on mean arterial pressure or heart rate (Figure 3).
Effect of stimulation of the ventral tegmental area on angiotensin and methoxamine
Intravenous injectin of 3, 10 and 30 ng kg−1 of angiotensin II caused a dose-dependent increase in mean arterial pressure and bradycardia (Table 2). These effects were not significantly different before and after micro-injection of either neurotensin or saline into the ventral tegmental area (Table 2). Intravenous injection of 20, 40 and 60 μg kg−1 of methoxamine caused a dose-dependent increase in mean arterial pressure and bradycardia (Table 2). As with angiotensin, the effects of methoxamine were similar before and after micro-injection of either neurotensin or saline into the ventral tegmental area (Table 2).
Table 2.
Effect of ventral tegmental area micro-injection of saline of neurotensin on mean arterial pressure and heart rate responses to intravenous injection of angiotensin II or methoxamine
Discussion
The most important finding of this study was that the micro-injection of neurotensin into the ventral tegmental area of conscious rats, while having little effect on resting mean arterial pressure, causes an enhancement of the pressor action of intravenously injected vasopressin. This effect was not seen in rats which had been pretreated with the dopamine D2 receptor antagonist raclopride which by itself did not influence the vasopressin pressor response. Thus, the action of raclopride is most likely to block an effect of neurotensin on central dopamine release and not merely to reduce the effect of vasopressin which, combined with a stimulation by neurotensin would have led to no net overall change. In addition, micro-injection of neurotensin into the ventral tegmental area did not alter the pressor action of angiotensin II or methoxamine. Taken together, these data suggest that neurotensin treatment stimulated dopaminergic cell bodies in the ventral tegmental area to release dopamine in an as yet unknown projection area of the mesolimbic dopamine system, resulting in larger increases in mean arterial pressure in response to vasopressin, but not angiotensin or methoxamine injections. Thus, at least in the present study, the effect of vasopressin on central cardiovascular regulation appears to involve a dopaminergic component originating in the ventral tegmental area. This interaction may be unique for vasopressin, at least in comparison with angiotensin or methoxamine under these experimental conditions.
Effects of vasopressin on blood pressure and cardiovascular reflexes
Neurotensin-induced stimulation of the ventral tegmental area augmented the effects of vasopressin, but not angiotensin II or methoxamine, on mean arterial pressure. This was not the result of an increase in vasopressin release as plasma vasopressin levels were not altered, but rather suggests that some unique aspects of the circulatory effects of vasopressin compared to the other vasopressor stimuli are susceptible to central dopaminergic modulation.
Administration of vasopressin to conscious animals induces a range of direct and indirect circulatory effects. Infusion of low doses of vasopressin induced preferential superior mesenteric vasoconstriction with little effect on mean arterial pressure, while higher doses caused mesenteric and hindquarter vasoconstriction and an increase in mean arterial pressure (Gardiner et al., 1988; 1999). These direct, vascular effects of vasopressin injections are associated with several reflex changes in cardiac output and sympathetic vasomotor tone (Bennett & Gardiner, 1986; Zhang et al., 1992). Direct measurements have shown decreased splanchnic and renal sympathetic activity in response to vasopressin treatment (Peuler et al., 1990; Veelken et al., 1989) which would counteract the direct vasoconstrictor action of this peptide. Indeed, baroreceptor denervation caused a marked leftward shift in the dose-response curve of vasopressin on mean arterial pressure (see Cowley et al., 1983). There has been considerable debate about whether in rats the indirect, reflex-induced effects of vasopressin are quantitatively different from those of other vasopressor stimuli, e.g. noradrenaline or angiotensin II (Bennett & Gardiner, 1986). For example, it was shown that administration of vasopressin induced reflex bradycardia and a fall in renal sympathetic nerve activity, but that only the effects on heart rate were greater than those evoked by phenylephrine (Peuler et al., 1990). By contrast, administration of vasopressin caused greater decreases in splanchnic sympathetic nerve activity as well as heart rate and cardiac output when compared to equipressor doses of methoxamine (Veelken et al., 1989). For the purpose of this discussion, it is sufficient to hypothesize that increased vasopressin levels in the blood recruit reflex mechanisms which are qualitatively different from those of other vasopressor stimuli. It could be at this unique part of the vasopressin feedback loop that mesolimbic dopaminergic stimulation interacts.
It is unlikely that vasopressin crosses the blood-brain-barrier to directly modulate either dopaminergic or hindbrain cardiovascular nuclei although some studies have suggested that peripheral administration of vasopressin may induce subtle centrally-mediated changes in cognition and learning (see Van Wimersma Greidanus et al., 1983). It is more likely that blood-borne vasopressin exerts a central action through the circumventricular organs, such as the area postrema in which vasopressin binding sites have been found (Phillips et al., 1988). Direct application of vasopressin on neurons in the area postrema caused neuronal activation in the nucleus tractus solitarius (Bishop & Hay, 1993; Migita et al., 1997), the primary relay nucleus for baroreceptor afferents in the brain (see Van Giersbergen et al., 1992). Lesions of the area postrema abolished the differential effect of vasopressin vs phenylephrine in inducing bradycardia (Peuler et al., 1990). One possible explanation for the present results could be that stimulation of the ventral tegmental area with neurotensin induces an effect similar to area postrema lesions, i.e. inhibits a feedback action of vasopressin on the brain. We recently showed that stimulation of the ventral tegmental area with DiMe-C7 caused an inhibition of the baroreceptor-heart rate reflex (Van den Buuse et al., 1998). However, similar experiments with neurotensin have not been performed yet. In addition, changes in baroreceptor-heart rate reflex do not necessarily reflect changes in reflex responses of sympathetic vasomotor or cardiac output and further experiments should focus on these mechanisms.
Micro-injection of neurotensin into the ventral tegmental area
In this study we used micro-injection of neurotensin to stimulate dopaminergic cell bodies in the ventral tegmental area. As reviewed by Kalivas (see Kalivas, 1993), high levels of neurotensin and neurotensin receptors have been found in the ventral tegmental area (Nicot et al., 1994; Uhl et al., 1979). Neurotensin receptors are located predominantly on dopaminergic cell bodies (see Kalivas, 1993; Palacios & Kuhar, 1981) and micro-injection of doses of neurotensin in the same range as used in the present study caused behavioural effects consistent with mesolimbic dopaminergic activation (see Kalivas, 1993; Kalivas et al., 1983). Local injection of neurotensin in the ventral tegmental area stimulated discharge activity of dopaminergic neurons (Seutin et al., 1989; Sotty et al., 1998) and stimulated dopamine release in mesolimbic terminal regions (see Kalivas, 1993). The effect of neurotensin on dopaminergic activity was longlasting (30–60 min) and showed little signs of desensitization (see Kalivas, 1993; Seutin et al., 1989). The dose-response curves for vasopressor agents in our experiments were obtained within 20 min after neurotensin injection.
The lack of effect of neurotensin on vasopressin release, compared to the substance P analogue DiMe-C7 (Cornish et al., 1997), is probably a reflection of the greater selectivity of neurotensin for dopaminergic cell bodies in the ventral tegmental area (see Kalivas, 1993). This allowed us to study the interaction of mesolimbic dopaminergic stimulation with the circulatory effects of vasopressin and other vasopressor agents without the confounding effects of elevated levels of endogenous vasopressin. In contrast to DiMe-C7 treatment, neurotensin injection produced only a small, non-significant increase in mean arterial presure which is unlikely to play a role in the enhancement of the vasopressin pressor response. Any effect on baseline blood pressure would have had a similar influence on the action of vasopressin, angiotensin, or methoxamine, unlike the selective effect we observed.
While we micro-injected neurotensin unilaterally into the ventral tegmental area, it is likely that with the injection volume used (1 μl) the contralateral side was stimulated as well. On the other hand, it is unlikely that the effect of neurotensin could be explained by an action on brain regions other than the ventral tegmental area. We previously showed that injection of the substance P analogue DiMe-C7 into, but not 2 mm above the ventral tegmental area, caused an increase in mean arterial pressure (Cornish & Van den Buuse, 1995). In addition, micro-injection of DiMe-C7 into the substantia nigra, adjacent to the ventral tegmental area, caused only minor effects on mean arterial pressure which were not influenced by pretreatment with the dopamine D2 receptor antagonist raclopride (Cornish & Van den Buuse, 1995). In the present study, the effect of neurotensin on the pressor action of vasopressin was not observed after pretreatment with raclopride, indicating that it too was dependent upon stimulation of dopaminergic cell bodies.
Functional implications
The interaction of central mesolimbic dopaminergic stimulation with the circulatory effects of vasopressin, but not other pressor agents, could have important subtle effects on cardiovascular control (see Van den Buuse, 1998). Activity of the mesolimbic dopamine system is stimulated by reward stimuli and stress (Abercrombie et al., 1989; Deutch et al., 1985). Interestingly, stress caused an increase in the concentration of neurotensin, but not other neuropeptides, in the ventral tegmental area (Deutch et al., 1987). This effect was associated with a selective increase in dopamine turnover in the ventral tegmental area and prefrontal cortex (Deutch et al., 1987), leading the authors to conclude that ‘neurotensin in the ventral tegmental area may be involved in environmentally elicited activation of certain mesotelencephalic dopamine neurons'. While further experiments are clearly needed, it is tempting to speculate on a role of frontal cortical dopamine projections in the effects of neurotensin-induced mesolimbic stimulation on cardiovascular control. Indeed, several studies have shown an involvement of cortical regions in the regulation of blood pressure (see Cechetto & Saper, 1990; Verberne & Owens, 1998). Stimulation of the prefrontal cortex induced effects on locomotor as well as cardiovascular components of the defence reaction (Haifa et al., 1989). Lesions of the prefrontal cortex reduce baroreflex control of heart rate by an effect on the slope, rather than the maximal responses of the blood pressure-heart rate relationship (Verberne et al., 1987). Micro-injection of DiMe-C7 into the ventral tegmental area induced a similar reduction in cardiac baroreflex sensitivity, an effect which was not observed after pretreatment with raclopride (Van den Buuse et al., 1998). However, while the ventral tegmental area projects to several other brain regions which play a role in cardiovascular regulation, such as the amygdala, bed nucleus of the stria terminalis and islands of Calleja (see Van den Buuse, 1998), it is too early to attribute the present effects to any of its projections without further experiments involving localized lesions or antagonist micro-injections. In any case, the ventral tegmental area has few projections to hindbrain cardiovascular regions (Lindvall & Björklund, 1974; Oades & Halliday, 1987; Swanson, 1982), making it conceivable that the present effects are mediated indirectly through its forebrain projections.
Conclusion
We found that stimulation of the ventral tegmental area of conscious rats with neurotensin caused selective enhancement of the effect of intravenous administration of vasopressin on mean arterial pressure. This effect was not observed in animals pretreated with raclopride, suggesting it was mediated by central dopamine release. These results are in line with our hypothesis (Cornish et al., 1997; see also Van den Buuse, 1998) which suggested a role of the mesolimbic/mesocortical system in modulating the circulatory effects of vasopressin. Whereas the pressor action of vasopressin is normally inhibited by its own feedback action on the brain, thus favouring its antidiuretic action, central dopaminergic stimulation, either by stress or reward stimuli (see Van den Buuse, 1998) would shift this balance towards a pressor action. The functional relevance of this shift could be to support or facilitate blood supply to relevant body parts in the face of environmental stimuli. As such, the mesolimbic/mesocortical system integrates the processing of sensory and behavioural information with maintenance of cardiovascular homeostasis.
Abbreviations
- ANOVA
analysis of variance
- MAP
mean arterial pressure
References
- ABERCROMBIE E.D., KEEFE K.A., DIFRISCHIA D.S., ZIGMOND M.J. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial prefrontal cortex. J. Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- BENNETT T., GARDINER S.M. Influence of exogenous vasopressin on baroreflex mechanisms. Clin. Sci. 1986;70:307–315. doi: 10.1042/cs0700307. [DOI] [PubMed] [Google Scholar]
- BISHOP V.S., HAY M. Involvement of the area postrema in the regulation of sympathetic outflow to the cardiovascular system. Front Neuroendocrinol. 1993;14:57–75. doi: 10.1006/frne.1993.1003. [DOI] [PubMed] [Google Scholar]
- CECHETTO D.F., SAPER C.B. Role of the cerebral cortex in autonomic function Central regulation of autonomic functions 1990Oxford: Oxford University Press; 208–223.eds. Loewy, A.D. & Spyer, K.M. [Google Scholar]
- CORNISH J.L., VAN DEN BUUSE M. Pressor responses to electrical and chemical stimulation of the rat brain A10 dopaminergic system. Neurosci. Lett. 1994;176:142–146. doi: 10.1016/0304-3940(94)90068-x. [DOI] [PubMed] [Google Scholar]
- CORNISH J.L., VAN DEN BUUSE M. Stimulation of the rat mesolimbic dopaminergic system produces a pressor response which is mediated by dopamine D-1 and D-2 receptor activation and the release of vasopressin. Brain Res. 1995;701:28–38. doi: 10.1016/0006-8993(95)00967-x. [DOI] [PubMed] [Google Scholar]
- CORNISH J.L., VAN DEN BUUSE M. Regional expression of c-fos in rat brain following stimulation of the ventral tegmental area. Neurosci. Lett. 1996;220:17–20. doi: 10.1016/s0304-3940(96)13222-5. [DOI] [PubMed] [Google Scholar]
- CORNISH J.L., WILKS D.P., VAN DEN BUUSE M. A functional interaction between the brain mesolimbic dopaminergic system and vasopressin release in the regulation of blood pressure. Neuroscience. 1997;81:69–78. doi: 10.1016/s0306-4522(97)00157-7. [DOI] [PubMed] [Google Scholar]
- COWLEY A.W., LIARD J.F. Vasopressin and arterial pressure regulation (Special lecture) Hypertension. 1988;11:I25–I32. doi: 10.1161/01.hyp.11.2_pt_2.i25. [DOI] [PubMed] [Google Scholar]
- COWLEY A.W., QUILLEN E.W., SKELTON M.M. Role of vasopressin in cardiovascular regulation. Fed. Proc. 1983;42:3170–3176. [PubMed] [Google Scholar]
- DEUTCH A.Y., BEAN A.J., BISSETTE G., NEMEROFF C.B., ROBBINS R.J., ROTH R.H. Stress-induced alterations in neurotensin, somatostatin and corticotropin-releasing factor in mesotelencephalic dopamine system regions. Brain Res. 1987;417:350–354. doi: 10.1016/0006-8993(87)90462-8. [DOI] [PubMed] [Google Scholar]
- DEUTCH A.Y., TAM S.Y., ROTH R.H. Footshock and conditioned stress increase 3,4-dihydroxyphenylacetic acid (DOPAC) in the ventral tegmental area but not substantia nigra. Brain Res. 1985;333:143–146. doi: 10.1016/0006-8993(85)90134-9. [DOI] [PubMed] [Google Scholar]
- DUNNETT S.B., ROBBINS T.W. The functional role of mesotelencephalic dopamine systems. Biol. Rev. 1992;67:491–518. doi: 10.1111/j.1469-185x.1992.tb01191.x. [DOI] [PubMed] [Google Scholar]
- EISON A.S., EISON M.A., IVERSEN S.D. The behavioural effects of a novel substance P analogue following infusion into the ventral tegmental area or substantia nigra of rat brain. Brain Res. 1982a;238:137–152. doi: 10.1016/0006-8993(82)90777-6. [DOI] [PubMed] [Google Scholar]
- EISON A.S., IVERSEN S.D., SANDBERG B.E.B., WATSON S.P., HANLEY M.R., IVERSEN L.L. Substance P analogue, DiMe-C7: evidence for stability in rat brain and prolonged central actions. Science. 1982b;215:188–190. doi: 10.1126/science.6171884. [DOI] [PubMed] [Google Scholar]
- ELLIOTT P.J., ALPERT J.E., BANNON M.J., IVERSEN S.D. Selective activation of mesolimbic and mesocortical dopamine metabolism in rat brain by infusion of a stable substance P analogue into the ventral tegmental area. Brain Res. 1986;363:145–147. doi: 10.1016/0006-8993(86)90667-0. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., BENNETT T., COMPTON A.M. Regional hemodynamic effect of neuropeptide Y, vasopressin and angiotensin II in conscious, unrestrained, Long Evans and Brattleboro rats. J. Auton. Nerv. System. 1988;24:15–27. doi: 10.1016/0165-1838(88)90131-2. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., CROMPTON A.M., BENNETT T. Regional hemodynamic effect of vasopressin infusion in conscious, unrestrained, Brattleboro rats. Brit. J. Pharmacol. 1989;97:147–152. doi: 10.1111/j.1476-5381.1989.tb11935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAIFA A., MASKATI A., ZBROZYNA A.W. Stimulation in prefrontal cortex area inhibits cardiovascular and motor components of the defense reaction in rats. J. Auton. Nerv. Syst. 1989;28:117–126. doi: 10.1016/0165-1838(89)90084-2. [DOI] [PubMed] [Google Scholar]
- HALL H., KÖHLER C., GAWELL L., FARDE L., SEDVALL G. Raclopride, a new selective ligand for the dopamine-D2 receptors. Progr. Neuro-Psychopharmacol. Biol. Psychiatry. 1988;12:559–568. doi: 10.1016/0278-5846(88)90001-2. [DOI] [PubMed] [Google Scholar]
- KALIVAS P.W. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res. Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- KALIVAS P.W., BURGESS S.K., NEMEROFF C.B., PRANGE A.J. Behavioural and neurochemical effects of neurotensin microinjection into the ventral tegmental area of the rat. Neuroscience. 1983;8:495–505. doi: 10.1016/0306-4522(83)90195-1. [DOI] [PubMed] [Google Scholar]
- KALIVAS P.W., SORG B.A., HOOKS M.S. The pharmacology and neural circuitry of sensitization to psychostimulants. Behav. Pharmacol. 1993;4:315–334. [PubMed] [Google Scholar]
- LE MOAL M., SIMON H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol. Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- LINDVALL O., BJÖRKLUND A. The organization of the ascending catecholamine neuron systems in the rat brain (as revealed by the glyoxylic acid fluorescence method) Acta. Physiol. Scand. 1974. pp. 1–48. [PubMed]
- MIGITA K., HORI N., MANAKO J., SAITO R., TAKANO Y., KAMIYA H. Effects of arginine-vasopressin on neuronal interaction from the area postrema to the nucleus tractus solitarii in rat brain slices. Neurosci. Lett. 1979;256:45–48. doi: 10.1016/s0304-3940(98)00753-8. [DOI] [PubMed] [Google Scholar]
- NICOT A., ROSTENE W., BEROD A. Neurotensin receptor expression in the rat forebrain and midbrain: a combined analysis by in situ hybridisation and receptor autoradiography. J. Comp. Neurol. 1994;341:407–419. doi: 10.1002/cne.903410310. [DOI] [PubMed] [Google Scholar]
- OADES R.D., HALLIDAY G.M. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. Rev. 1987;12:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- PALACIOS J.M., KUHAR M.J. Neurotensin receptors are located on dopamine-containing neurones in rat midbrain. Nature. 1981;294:587–589. doi: 10.1038/294587a0. [DOI] [PubMed] [Google Scholar]
- PEULER J.D., EDWARDS G.L., SCHMID P.G., JOHNSON A.K. Area postrema and differential reflex effects of vasopressin and phenylephrine in rats. Am. J. Physiol. 1990;258:H1255–H1259. doi: 10.1152/ajpheart.1990.258.4.H1255. [DOI] [PubMed] [Google Scholar]
- PHILLIPS P.A., ABRAHAMS J.M., KELLY J., PAXINOS G., GRZONKA Z., MENDELSOHN F.A.O., JOHNSTON C.I. Localization of vasopressin binding sites in rat brain by in vitro autoradiography using a radioiodinated V-1 receptor antagonist. Neuroscience. 1988;27:749–761. doi: 10.1016/0306-4522(88)90180-7. [DOI] [PubMed] [Google Scholar]
- SCHMID P.G., SHARABI F.M., GUO G.B., ABBOUD F.M., THAMES M.D. Vasopressin and oxytocin in the neural control of the circulation. Fed. Proc. 1984;43:97–102. [PubMed] [Google Scholar]
- SEUTIN L., MASSOTTE L., DRESSA A. Electrophysiological effects of neurotensin on dopaminergic neurons of the ventral tegmental area of the rat in vitro. Neuropharmacology. 1989;28:949–954. doi: 10.1016/0028-3908(89)90194-9. [DOI] [PubMed] [Google Scholar]
- SOTTY F., SOULIERE F., BRUN P., CHOUVET G., STEINBERG R., SOUBRIE P., RENAUD B., SUAUD-CHAGNY M.F. Differential effects of neurotensin on dopamine release in the caudal and rostral nucleus accumbens: A combined in vivo electrochemical and electrophysiological study. Neuroscience. 1998;85:1173–1182. doi: 10.1016/s0306-4522(97)00691-x. [DOI] [PubMed] [Google Scholar]
- SWANSON L.W. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- UHL G.R., GOODMAN R.R., SNYDER S.H. Neurotensin-containing cell bodies, fibres and nerve terminals in the brain stem of the rat: immunohistochemical mapping. Brain Res. 1979;167:77–91. doi: 10.1016/0006-8993(79)90264-6. [DOI] [PubMed] [Google Scholar]
- VAN DEN BUUSE M. Central effects of quinpirole on blood pressure of spontaneously hypertensive rats. J. Pharmacol. Exp. Ther. 1992;262:303–311. [PubMed] [Google Scholar]
- VAN DEN BUUSE M. Role of the mesolimbic dopamine system in cardiovascular homeostasis. Stimulation of the ventral tegmental area modulates the effect of vasopressin on blood pressure in conscious rats. Clin. Exp. Pharmacol. Physiol. 1998;25:661–668. doi: 10.1111/j.1440-1681.1998.tb02273.x. [DOI] [PubMed] [Google Scholar]
- VAN DEN BUUSE M., MORTON S.J., CORNISH J.L., HEAD G.A. Prolonged effects of quinpirole on cardiovascular regulation. J. Pharmacol. Exp. Ther. 1996;277:473–483. [PubMed] [Google Scholar]
- VAN DEN BUUSE M., WILKS D.P., CORNISH J.C. Inhibition of cardiac baroreflex sensitivity after central dopaminergic stimulation. Clin. Exp. Pharmacol. Physiol. 1998;25:624–626. doi: 10.1111/j.1440-1681.1998.tb02264.x. [DOI] [PubMed] [Google Scholar]
- VAN GIERSBERGEN P.L.M., PALKOVITS M., DE JONG W. Involvement of neurotransmitters in the nucleus tractus solitarii in cardiovascular regulation. Physiol. Rev. 1992;72:789–824. doi: 10.1152/physrev.1992.72.3.789. [DOI] [PubMed] [Google Scholar]
- VAN WIMERSMA GREIDANUS T.B., VAN REE J.M., DE WIED D. Vasopressin and memory. Pharmacol. Ther. 1983;20:437–458. doi: 10.1016/0163-7258(83)90036-0. [DOI] [PubMed] [Google Scholar]
- VEELKEN R., DANCKWART L., ROHMEISS P., UNGER T. Effects of intravenous AVP on cardiac output, mesenteric hemodynamics, and splanchnic nerve activity. Am. J. Physiol. 1989;257:H658–H664. doi: 10.1152/ajpheart.1989.257.2.H658. [DOI] [PubMed] [Google Scholar]
- VERBERNE A.J.M., LEWIS S.J., WORLAND P.J., BEART P.M., JARROT B., CHRISTIE M.J., LOUIS W.J. Medial prefrontal cortex lesions modulate baroreflex sensitivity in the rat. Brain Res. 1987;426:243–249. doi: 10.1016/0006-8993(87)90878-x. [DOI] [PubMed] [Google Scholar]
- VERBERNE A.J.M., OWENS N.C. Cortical modulation of the cardiovascular system. Prog. Neurobiol. 1998;54:149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- WEBB R.L., OSBORN J.W., COWLEY A.W. Cardiovascular actions of vasopressin: baroreflex modulation in the conscious rat. Am. J. Physiol. 1986;251:H1244–H1251. doi: 10.1152/ajpheart.1986.251.6.H1244. [DOI] [PubMed] [Google Scholar]
- WOODS R.L., JOHNSTON C.I. Contribution of vasopressin to the maintenance of blood pressure during dehydration. Am. J. Physiol. 1983;245:F615–F621. doi: 10.1152/ajprenal.1983.245.5.F615. [DOI] [PubMed] [Google Scholar]
- ZHANG X., ABDEL-RAHMAN A.R.A., WOOLES W.R. Vasopressin receptors in the area postream differentially modulate baroreceptor responses in rats. Eur. J. Pharmacol. 1992;222:81–91. doi: 10.1016/0014-2999(92)90466-h. [DOI] [PubMed] [Google Scholar]


