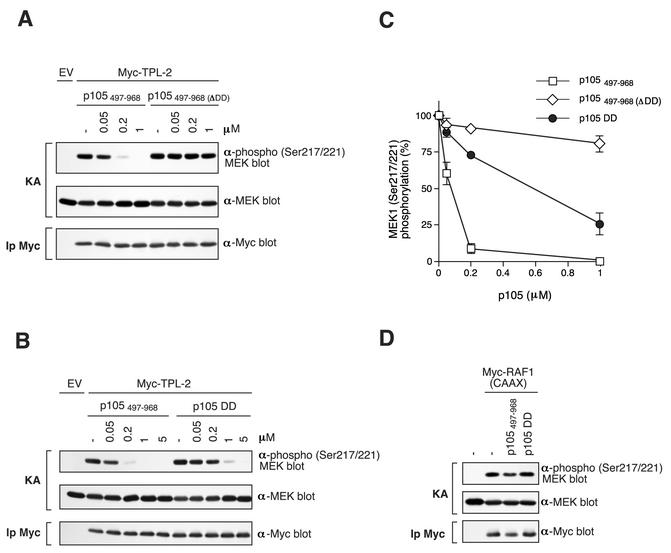
FIG. 7.
TPL-2 MEK kinase activity is inhibited by p105 in vitro. (A and B) Myc-TPL-2 was isolated by immunoprecipitation from lysates of transfected 293 cells and then preincubated with the different amounts of the indicated recombinant p105 proteins or control buffer (−). In vitro kinase assays (KAs) were performed with GST-MEK1(K207A) as a substrate and phosphorylation determined by Western blotting of reaction mixtures and probing with an anti-phospho-MEK1/2 Ser217/Ser221 antibody. Equal loading of GST-MEK1(K207A) protein was confirmed by reprobing blots with anti-MEK1/2 antibody. Western blotting of anti-Myc immunoprecipitates (Ip) demonstrated that similar amounts of TPL-2 were assayed in each reaction (lower panel). (C) MEK1 phosphorylation in replicates of the experiments shown in A and B was quantified by laser densitometry (n = 3). Data are presented as percentages of control MEK kinase activity. (D) Myc-Raf1(CAAX) was immunoprecipitated from lysates of transfected 293 cells and MEK kinase activity assayed as in panel A. Recombinant p105 protein was added to a final concentration of 5 μM.