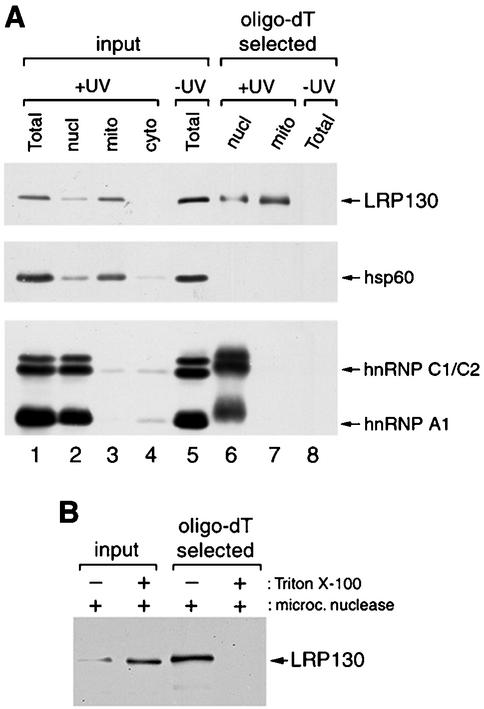
FIG. 3.
LRP130 is bound to mitochondrial polyadenylated RNAs in vivo. (A) Nuclear and mitochondrial fractions from UV-irradiated HeLa cells and total extract from control cells were fractionated by oligo(dT) chromatography under protein-denaturing conditions to select polyadenylated RNAs with cross-linked proteins (lanes 6 to 8). Proteins in the selected RNPs were released by digestion with RNase and analyzed by immunoblotting with antibodies against LRP130, hsp60, hnRNP C1/C2, and hnRNP A1. Abbreviations are as for Fig. 2. (B) The mitochondrial fraction from UV-irradiated cells was treated with micrococcal nuclease in the presence or absence of Triton X-100. Cross-linked RNP complexes were selected by oligo(dT) chromatography, and bound proteins were released and analyzed as described for panel A by using the anti-LRP130 MAb 4C12. Input lanes contain approximately 1% of the material loaded on the oligo(dT) columns. The smaller amount of LRP130 in the lane corresponding to mitochondria treated in the absence of Triton X-100 is most likely due to the fact that, under these conditions, the nuclease cannot gain access to the LRP130-cross-linked RNAs. The cross-linked LRP130-RNA complexes that resist nuclease digestion would, in turn, be too large to enter the gel.