FIG. 1.
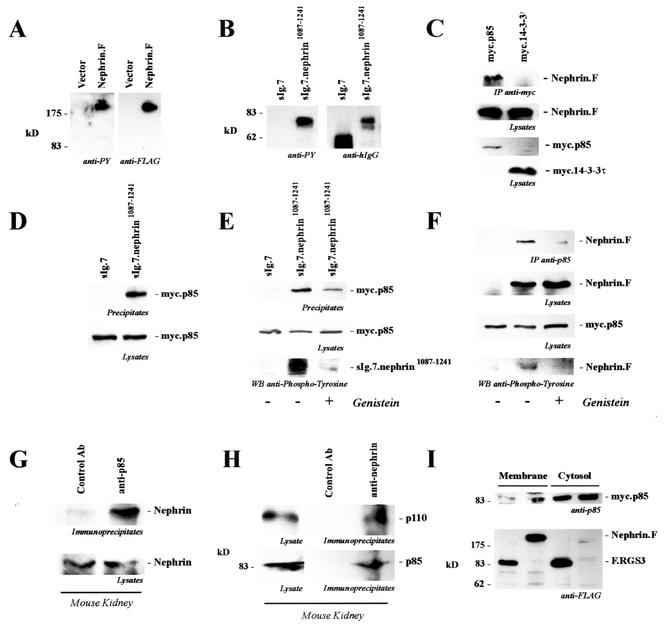
The carboxy-terminal cytoplasmic tail of nephrin interacts with p85. (A) C-terminally FLAG-tagged full-length nephrin (Nephrin.F) was expressed in HEK 293T cells and immunoprecipitated with anti-FLAG antibody. Tyrosine phosphorylation was detected with the specific antiphosphotyrosine antibody 4G10 (anti-PY; left). On the right is shown a reprobe with anti-FLAG antibody. (B) The cytoplasmic tail of nephrin was fused to the CH2 and CH3 domains of human IgG1, followed by the transmembrane region of CD7 (sIg.7.nephrin1087-1241), and precipitated with protein G-Sepharose beads. The CH2 and CH3 domains of human IgG1, followed by the transmembrane region of CD7 without a cytoplasmic tail, served as the control (sIg.7). Tyrosine phosphorylation was detected with the antiphosphotyrosine-specific antibody 4G10 (anti-PY; left). On the right are shown expression levels of the constructs (reprobe with anti-human IgG antibody). (C) C-terminally FLAG-tagged full-length nephrin (Nephrin.F) was coexpressed with myc-tagged p85 or 14-3-3τ. After immunoprecipitation (IP) with the anti-myc antibody, the immobilized nephrin was detected with the anti-FLAG antibody (top). The middle and lower blots show expression of the proteins in cell lysates. (D and E) myc.p85 was coexpressed with the constructs described for panel B. After precipitation with protein G, immobilized p85 was detected with a specific anti-p85 antibody. Prior to lysis and immunoprecipitation, cells were treated with solvent (−) or genistein (50 μM; 30 min) (+) as indicated. (D) Top, precipitates; bottom, lysates stained for p85. (E) Top, precipitates stained for p85; middle, lysates stained for p85; bottom, nephrin in precipitates stained for phosphotyrosine. (F) myc.p85 was coexpressed with C-terminally FLAG-tagged full-length nephrin (Nephrin.F). After precipitation of p85, nephrin was detected with anti-FLAG antibody and tyrosine phosphorylation of nephrin was detected with antiphosphotyrosine antibody. Cells were treated with solvent or genistein (50 μM, 30 min) prior to lysis and immunoprecipitation. Top, precipitates stained for nephrin; upper middle, lysates stained for nephrin; lower middle, lysates stained for p85; bottom, nephrin in precipitates stained for phosphotyrosine. (G) To show endogenous interaction, mouse kidneys were freshly isolated and perfused in situ with ice-cold phosphate-buffered saline and subjected to glass-glass homogenization in a CHAPS lysis buffer. The precleared lysates were prepared by extensive centrifugation and preabsorption and subsequently divided into two portions. One half was immunoprecipitated with a control antibody (Ab) (anti-HA antibody), and the other half was precipitated with an anti-p85 antibody. Immobilized nephrin was detected with a nephrin-specific antiserum. (H) To confirm endogenous interaction, precleared lysates of mouse kidney were divided into two portions. One half was immunoprecipitated with a control antibody (anti-HA antibody), and the other half was precipitated with an antinephrin antibody. Immobilized p85 was detected with a p85-specific antiserum (bottom). On top is shown the coprecipitating enzymatically active p110 subunit of PI3 kinase, indicating that nephrin interacts with the active heterodimer of PI3K. (I) myc.p85-expressing HEK 293T cells were transfected with FLAG-tagged nephrin or with FLAG-tagged RGS3 (F.RGS3) as a control protein. Recruitment of myc.p85 to the membrane compartment was monitored by preparing a 100,000 × g pellet (P100 [Membrane]) and a 100,000 × g supernatant fraction (S100 [Cytosol]).