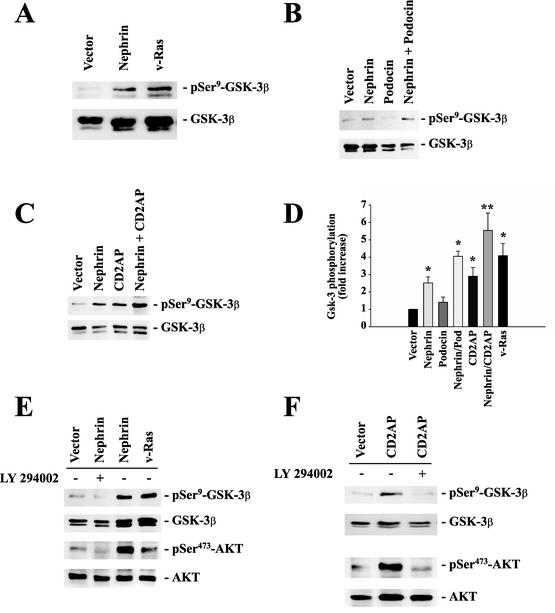
FIG. 4.
Nephrin, CD2AP, and podocin cooperatively activate the PI3K/AKT pathway. To monitor AKT activity, phosphorylation of GSK-3β at serine-9 was measured in HEK 293T cells transfected with identical amounts of plasmid DNA, as indicated (A, B, C, E, and, F, top). The cells were cotransfected with 4 μg of myc.AKT and 4 μg of GSK-3β; with 8 μg of vector for vector alone and 4 μg of expression plasmid plus 4 μg of vector for nephrin, podocin, CD2AP, or v-Ras alone; and with 4 plus 4 μg of plasmid DNA for nephrin plus CD2AP or nephrin plus podocin. This approach ensured equal expression levels of the relevant cDNAs and allowed statistical analysis of several independent experiments. Equal loading and comparable expression of AKT and GSK-3β were confirmed by reprobing the blots with nonphosphospecific anti-AKT and anti-GSK-3β antisera (middle and bottom) and with the relevant antisera against podocin, nephrin, and CD2AP (not shown). (D) The degree of phosphorylation was quantified by densitometry of nonsaturated radiographs with NIH Image software. Depicted is the statistical analysis of 10 independent experiments (*, P < 0.05; **, P < 0.01; n = 10). The error bars indicate SEM. (E and F) Incubation of cells with (+) the specific PI3K inhibitor LY 294002 (25 μM; 30 min) abrogated nephrin-CD2AP-stimulated AKT activation and AKT-mediated GSK-3β phosphorylation.