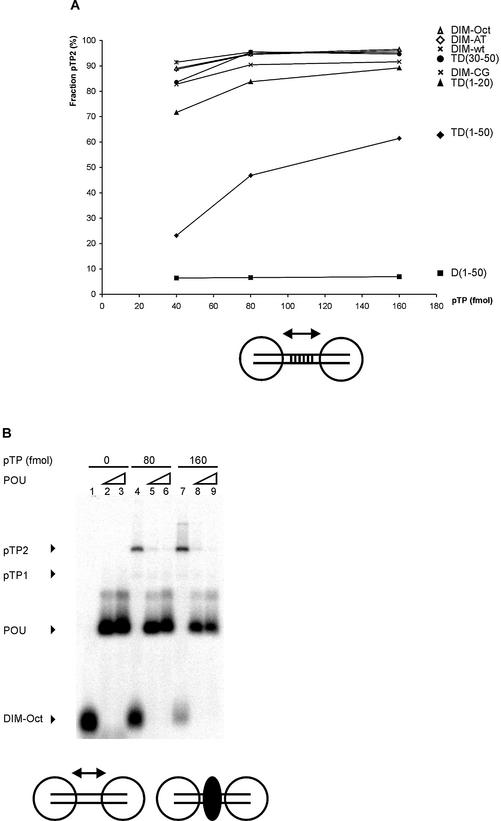
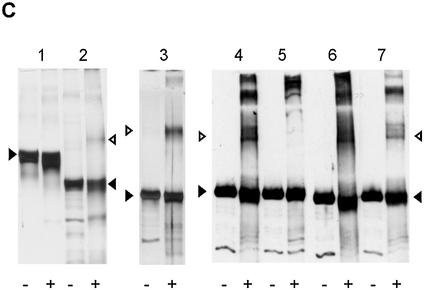
Figure 3.
Cooperative pTP binding is most likely mediated by protein/protein contacts. (A) Increasing amounts of pTP were incubated with different DNA duplexes consisting of TD(1–20) with the following 10 bp long insertions between bp 10 and 11. DIM-wt, Ad ori bp 11–20; DIM-AT, AT-rich; DIM-CG, CG-rich; DIM-Oct, octamer site. Amount of pTP2 complex as fraction of the total amount of shifted pTP/DNA complex is plotted against pTP concentration. Values are averaged over two experiments; error margins were <10% fraction pTP2. Indicated schematically at the bottom of the figure are two pTP molecules (large circles) binding to the DIM-wt probe with the variable insertion sequence indicated by parallel lines. (B) Binding of pTP to DIM-Oct was studied using EMSA. 0, (lanes 1–3), 80 (4–6) or 160 (7–9) fmol pTP was incubated with DNA in the presence of 5 (lanes 2, 5 and 8) or 10 (lanes 3, 6 and 9) ng Oct-1 POU domain. Indicated schematically at the bottom of the figure are two pTP molecules bound to DIM-Oct in the absence (left) and presence (right) of Oct-1 POU (black oval) binding the insertion sequence in the middle of the DIM-Oct probe. (C) EDC crosslinking analysis of protein/protein interaction. An aliquot of 5 µM of each protein was incubated without (– lanes) or with (+ lanes) 10 mM EDC, separated by SDS–PAGE (lanes 1 and 2, 10%; lanes 3–7, 7.5%) and detected by silver-staining (lanes 1–3) or western blotting using an α-pTP polyclonal antibody (lanes 4–7). Lane 1, ovalbumin; lane 2, NFI DNA binding domain; lane 3, Ad5 pTP; lane 4, Ad5 pTP; lane 5, Ad5 pTP + pol; lane 6, Ad4 pTP; lane 7, Ad5 pTP + 7 µM TD(1–20). Filled triangles indicate monomers, open triangles indicate dimers formed after EDC treatment.