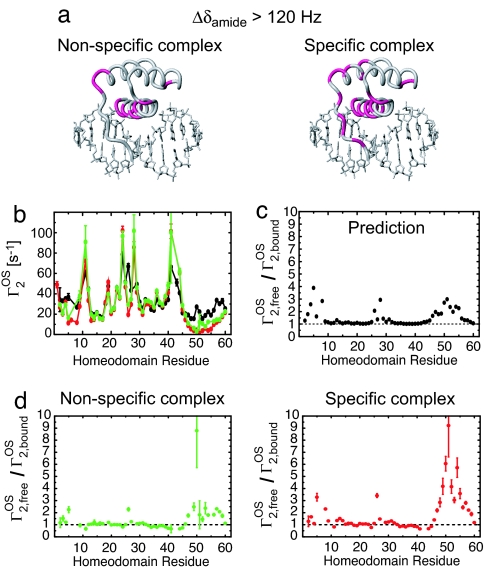
Fig. 3.
The HoxD9 homeodomain binds to nonspecific DNA by using the same interface as that in the specific complex. (a) Residues exhibiting a large (>120 Hz at a 1H frequency of 600 MHz) 1HN/15N chemical shift perturbation, Δδamide [defined as (ΔδH2 + ΔδN2)1/2 in Hz], upon complex formation are colored in lilac on the structure of the specific complex. (b) 1HN-Γ2 PREs (Γ2OS) arising from 3 mM paramagnetic cosolute Gd-DTPA-BMA. Data measured on the nonspecific complex, specific complex and free protein are shown in green, red, and black, respectively. (c) Predicted profile of Γ2,freeOS/Γ2,boundOS for the specific complex. The ratio was predicted from the structures by using a grid-based approach (20, 21). The radius of the Gd–DTPA-BMA molecule was set to 3.5 Å. The correlation time for the PRE was assumed to be dominated by translational diffusion of the paramagnetic cosolute molecule and virtually identical for the free and bound states of the protein. (d) Experimental Γ2,freeOS/Γ2,boundOS ratios for the nonspecific and specific complexes. The binding interface can be identified from regions with Γ2,freeOS/Γ2,boundOS > 1.