Abstract
Acute lymphoblastic leukemia (ALL) is a clonal disease that evolves through the accrual of genetic rearrangements and/or mutations within the dominant clone. The TEL-AML1 (ETV6-RUNX1) fusion in precursor-B (pre-B) ALL is the most common genetic rearrangement in childhood cancer; however, the cellular origin and the molecular pathogenesis of TEL-AML1-induced leukemia have not been identified. To study the origin of TEL-AML1-induced ALL, we generated transgenic zebrafish expressing TEL-AML1 either ubiquitously or in lymphoid progenitors. TEL-AML1 expression in all lineages, but not lymphoid-restricted expression, led to progenitor cell expansion that evolved into oligoclonal B-lineage ALL in 3% of the transgenic zebrafish. This leukemia was transplantable to conditioned wild-type recipients. We demonstrate that TEL-AML1 induces a B cell differentiation arrest, and that leukemia development is associated with loss of TEL expression and elevated Bcl2/Bax ratio. The TEL-AML1 transgenic zebrafish models human pre-B ALL, identifies the molecular pathways associated with leukemia development, and serves as the foundation for subsequent genetic screens to identify modifiers and leukemia therapeutic targets.
Keywords: stem cell, translocation, childhood cancer, genetics
The TEL-AML1 fusion generated by the t(12, 21)(p13;q22) chromosomal translocation is present in 25% of childhood pre-B acute lymphoblastic leukemia (ALL), making it the most common genetic rearrangement in childhood cancer (1–3). The translocation fuses the first five exons of the Ets transcription factor TEL (also known as ETV6) in-frame to nearly the entire AML1 gene (also known as RUNX1). Retrospective studies in twins with pre-B ALL, as well as Guthrie cards studies from 567 normal newborns (4), reveal that the TEL-AML1 fusion occurs in utero, with a protracted time course for leukemia development (5, 6).
Murine studies involving TEL-AML1 suggest that this fusion protein confers a low transforming ability. Transgenic mice expressing TEL-AML1 from the Ig heavy chain promoter (Eμ) did not develop any hematological disorder (7). Mice transplanted with bone marrow cells transduced with retroviral vectors expressing TEL-AML1 developed a preleukemic state without occult leukemia (8–10). The incidence of leukemia in such mice increased only in the presence of cooperating mutations (11).
The cell initially transformed by TEL-AML1 remains to be elucidated; however, in ALL patients, the TEL-AML1 fusion event precedes differentiation of lymphoid progenitors to pre-B cells (12). This finding confines the origin of pre-B ALL to a B-lineage restricted progenitor(s) (4) or a multipotent hematopoietic stem cell (HSC) with preferential B-lymphoid clonal expansion (13).
We used the zebrafish to study TEL-AML1 leukemogenesis for several reasons. First, the zebrafish has well conserved genetic processes controlling hematopoesis (14, 15). Second, zebrafish develop tumors that are histologically similar to human tumors (16–20). The lymphoid expression of mouse c-Myc led to the development of T cell leukemia in 6–13% of the injected fish (18) and progeny within 2–5 months (19). Also, zebrafish expression of the TEL-JAK2 (20), or the human AML1-ETO (17), fusion cDNAs led to hematopoietic perturbation (17, 20). Third, the highly conserved TEL and AML1 sequences among vertebrates, with 93% homology between Teleost fish and human TEL Ets domain (ref. 21 and H.S. and D.D.H., unpublished data), and 99% homology between zebrafish and human AML1 Runt domain (17, 22), makes the zebrafish an attractive model to study TEL-AML1-associated leukemia.
We generated transgenic zebrafish expressing the TEL-AML1 fusion. Acute lymphoblastic leukemia developed after long latency, and only when TEL-AML1 was expressed at the noncommitted progenitor level. This transgenic model provides the opportunity to study the multiple genetic events associated with TEL-AML1 induced leukemia.
Results
TEL-AML1 Transgenic Zebrafish Lines.
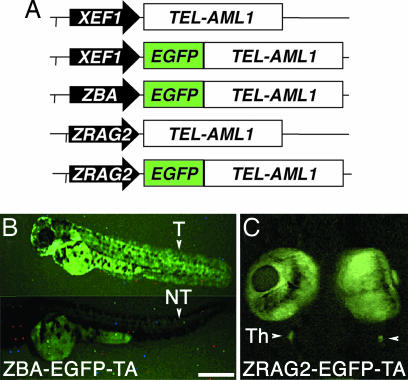
Three different promoters were used to express TEL-AML1, either alone or fused to EGFP, in a ubiquitous or tissue-specific manner (Fig. 1A). Both the Xenopus elongation factor 1α (XEF) and the zebrafish β-actin (ZBA) promoters direct expression to all lineages (23, 24), whereas the zebrafish recombination activation gene 2 (RAG2) promoter restricts expression to B and T cell lymphoid progenitors (25). Before microinjections in fertilized zebrafish embryos, the expression of the appropriate molecular weight TEL-AML1 or EGFP-TEL-AML1 proteins was confirmed by Western blotting of the in vitro transcription and translation products (Fig. 5, which is published as supporting information on the PNAS web site).
Fig. 1.
TEL-AML1 transgenic zebrafish with ubiquitous and lymphoid-restricted expression. (A) Diagrams of the human TEL-AML1c (TA) cDNA, alone or fused in-frame to EGFP, expressed from the ubiquitous Xenopus elongation factor-1 (XEF) 0.7 kb, the zebrafish β-actin (ZBA) 4.5-kb promoters, or from the lymphoid zebrafish Recombination Activation Gene-2 (ZRAG2) 6.5-kb promoter. (B) Transgenic embryos (T) from the ZBA-EGFP-TA line expressing EGFP-TEL-AML1 at 3 dpf compared with nontransgenic (NT) sibling. Fish were oriented with anterior to the left and dorsal to the top (T) or dorsal to the bottom (NT). (C) Ventral view of a 7-dpf RAG2-EGFP-TA zebrafish with EGFP-TEL-AML1-labeled cells in the bilateral thymus (Th) (arrowheads). (Scale, 1 mm in B and 2 mm in C.)
Identification of Transgenic Founders and Establishment of Transgenic Lines.
To establish TEL-AML1 transgenic founders, linearized DNA constructs were microinjected into one- to two-cell stage embryos. Selected embryos were grown to maturity and crossed with wild-type fish, and genomic DNA from fertilized eggs was analyzed to identify germ-line founders. Thirteen founders were identified from the XEF-TEL-AML1 (XEF-TA) line, eight founders were identified from the XEF-EGFP-TEL-AML1 (XEF-EGFP-TA) line, 44 founders were identified from the ZBA-EGFP-TEL-AML1 (ZBA-EGFP-TA) line, six founders were identified from the RAG2-TEL-AML1 (RAG2-TA) line, and five founders were identified from the RAG2-EGFP-TEL-AML1 (RAG2-EGFP-TA) line. At least three founders per construct were crossed to wild-type, and the progeny were propagated and maintained for >2 years. Stable Mendelian transmission and expression of TEL-AML1 for all lines has been demonstrated over five generations.
Ubiquitous and Lymphoid TEL-AML1 Expression in Transgenic Zebrafish.
RT-PCR positive F1 fish were crossed to wild-type, the F2 progeny were typed, and the RT-PCR-positive fish (Fig. 6, which is published as supporting information on the PNAS web site) were grown to maturity and intercrossed. RT-PCR and fluorescent analysis of the F3 progeny from the XEF-TA, XEF-EGFP-TA, and the ZBA-EGFP-TA transgenic fish demonstrated ubiquitous mRNA expression. In both the RAG2-TA and RAG2-EGFP-TA lines, TEL-AML1 expression in lymphoid progenitors was only detected in the kidney and thymus (Fig. 6), the sites of B and T cell lymphopoiesis, respectively, in adult zebrafish (26). An average of 4.37% of kidney marrow progenitors from RT-PCR positive RAG2-EGFP-TA fish expressed EGFP by flow cytometry (data not shown). This level of expression is similar to the reported number of RAG2 expressing progenitors in zebrafish marrow (27).
The zebrafish β-actin promoter directed sufficient EGFP expression to allow visual selection of transgenic progeny (Fig. 1B), and to distinguish homozygous TEL-AML1 fish (TA/TA), subsequently confirmed to propagate EGFP-TEL-AML1 to all their progeny, from heterozygous (TA/WT) and wild-type (WT/WT) siblings based on fluorescence (Fig. 7, which is published as supporting information on the PNAS web site).
RAG2 lymphoid-specific expression was detected in cells of the small bilateral thymus by fluorescent microscopy at 7 days postfertilization (dpf) (Fig. 1C). Confocal microscopy confirmed EGFP-TEL-AML1 expression in mature fish (data not shown). The fluorescent expression of EGFP-TEL-AML1 from the XEF1 promoter frequently could not be visualized; therefore, in situ hybridization was used to confirm the ubiquitous mRNA expression (data not shown). Additionally, TEL-AML1 proteins were detected by Western blotting in transgenic fish (Fig. 8, which is published as supporting information on the PNAS web site), at levels comparable to those of the TEL-AML1 expressing human Reh cells (28).
Evidence of B Cell Differentiation Arrest in TEL-AML1 Transgenic Zebrafish.
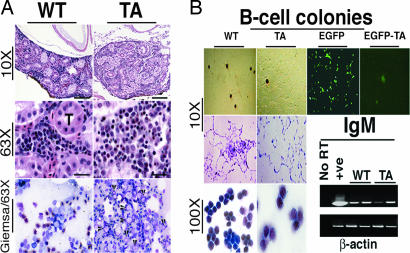
When TEL-AML1 was expressed ubiquitously, five XEF-TA, nine XEF-EGFP-TA, and 17 ZBA-EGFP-TA transgenic fish, ≈6% of the transgenic fish in these three lines (n = 31 of 545), developed fatal lymphoid hyperplasia that was detected as early as 4 weeks postfertilization (Fig. 2A). Lymphoid hyperplasia was defined by increased immature “blast like” lymphoid cells in peripheral blood, but below the level of 20% blasts required to diagnose leukemia, and without distal infiltration. The gross features in these fish included palor, cachexia, and extensive s.c. hemorrhage. Manual differential blood cell counts revealed that wild-type fish have, on average, 94% lymphocytes, 5% segmented heterophils, and 1% monocytes. The TEL-AML1 transgenic fish with lymphoid hyperplasia showed 10–15% immature blast-like cells, 75–80% lymphocytes, 1–4% heterophils, and 0–3% monocytes. Compared with wild-type fish, blood smears from 11 fish with lymphoid hyperplasia showed mild to moderate thrombocytopenia, and in three fish, thrombocytosis was present associated with many diploid prothrombocyte precursors. Histological sections of the kidney marrow from these fish revealed increased number of immature basophilic lymphoid cells, with nucleoli consistent with lymphoid progenitors, compared with wild-type fish (Fig. 2A).
Fig. 2.
Evidence of B-lymphoid differentiation arrest in TEL-AML1 transgenic zebrafish. (A) Lymphoid hyperplasia in TEL-AML1 transgenic zebrafish. Hematoxylin and eosin (H&E) staining of kidney marrow between the tubules (T) from wild-type (WT), and transgenic ZBA-EGFP-TA fish (TA) with lymphoid hyperplasia (×10 and ×63). Touch preps from transgenic fish stained with Giemsa (×63) show increased basophilic immature cells with nucleoli consistent with lymphoid progenitors (arrowheads), compared with wild-type. (B) In vitro, mitogen-induced B cell clonogenic assays indicate reduced colony numbers from TEL-AML1 (TA)-expressing XEF-TA transgenic fish, compared with wild-type (WT). Sorted EGFP-positive kidney progenitors from the ZBA-EGFP-TA transgenic fish developed significantly less EGFP-positive colonies (EGFP-TA) than from control fish (EGFP). May–Grunwald/Giemsa-stained B cell colonies from wild-type and transgenic cells, shown in low (×10) and high (×100) power. Colony cells from wild-type and TEL-AML1 transgenic cells (from two different colonies) expressed the constant region of IgM by RT-PCR.
We analyzed kidney marrow cells from each TEL-AML1 transgenic line (n = 10 per line), as well as wild-type and control RAG2-EGFP fish by flow cytometry (27). A 2- to 3-fold relative increase in the progenitor fraction of the TEL-AML1 transgenic marrow compared with wild-type and control marrow was observed (Table 1). Total cell counts showed a modest decrease in myeloid, and a slight increase of erythroid cells associated with the progenitor cell expansion. To assess the lymphoid proliferative potential of these precursors, kidney marrow progenitors were subjected to an in vitro mitogen-induced B cell clonogenic assay (29) (Fig. 2B and Table 1). After 3–5 days of culture with bacterial LPS, a polyclonal B cell activator, colonies of 50–350 cells developed. A linear relationship was found between the number of cells seeded and the number of colonies developed (data not shown). Colonies generated from the XEF-TA transgenic marrow were reduced by a factor of 10 compared with cells from wild-type fish (Fig. 2B and Table 1). The EGFP-expressing lymphoid progenitors from the ZBA-EGFP-TA, the RAG2-EGFP-TA transgenic fish, and control- ZBA- and RAG2-EGFP fish were cultured in the same B cell clonogenic assay. A small number of fluorescent colonies developed with cells from the ZBA-EGFP-TA transgenic fish compared with cells from the ZBA-EGFP control fish (Fig. 2B, EGFP-TA vs. EGFP). Consistent with the interpretation that TEL-AML1 expression impairs B cell maturation, EGFP expressing lymphoid progenitors remained at the single cell level (Fig. 2B, EGFP-TA) and failed to differentiate to mature B cell colonies under these conditions. The number of colonies generated from the RAG2-driven TEL-AML1 cells was not reduced (Experiment 2 in Table 1) compared with control EGFP cells, indicating the requirement to express TEL-AML1 at a level earlier than the committed lymphoid progenitors to elicit a differentiation arrest. The morphology of the colony cells, and the fact that they expressed the constant region of IgM by RT-PCR (Fig. 2B), suggests that the colonies developed from B lymphocytic progenitors. We attempted to develop an in vitro pre-B cell clonogenic assay using either recombinant human or murine IL-7, but zebrafish marrow progenitors were irresponsive to IL-7 stimuli, possibly due to the lack of a zebrafish IL-7 orthologue (H.S. and D.D.H., unpublished data).
Table 1.
Lineage distribution and B-lymphoid in vitro colony-forming cell activity of TEL-AML1 progenitor cells
| Zebrafish line | Lineage distribution by FACS, no. of cells (×104) (%) |
B cell colonies |
||||
|---|---|---|---|---|---|---|
| Erythroid | Myeloid | Lymphoid | Progenitor | n | Cells harvested per well (×103) | |
| Experiment 1 | ||||||
| Wild-type | 108 ± 17.3 (56.9 ± 9.1) | 38.4 ± 9.8 (20.9 ± 5.1) | 17.2 ± 7.3 (9.6 ± 3.8) | 8.7 ± 5.8 (4.8 ± 3.1) | 38 ± 9 | 140 |
| XEF-TA | 123 ± 28.3 (68.2 ± 15.7) | 30.0 ± 7.1 (16.7 ± 3.9) | 5.9 ± 3.8 (3.3 ± 2.1) | 30.2 ± 8.3 (14.2 ± 4.6)* | 3 ± 2* | 18 |
| XEF-EGFP-TA | 108 ± 15.9 (59.9 ± 8.8) | 38.4 ± 6.3 (18.9 ± 3.5) | 14.7 ± 8.8 (8.2 ± 4.8) | 20.5 ± 9.3 (11.4 ± 5.2)* | 7 ± 4* | 43 |
| ZBA-EGFP-TA | 106 ± 10.3 (58.7 ± 5.7) | 16.0 ± 7.4 (8.9 ± 4.1) | 6.8 ± 4.2 (3.8 ± 2.3) | 41.9 ± 16.3 (23.3 ± 9.0)* | 14 ± 9* | 67 |
| Experiment 2 | ||||||
| RAG2-EGFP-TA | 110 ± 12.9 (58.1 ± 7.2) | 32.9 ± 6.0 (18.3 ± 3.3) | 16.2 ± 6.3 (9.0 ± 3.5) | 10.2 ± 6.1 (5.4 ± 3.4) | 246 ± 23 | 1,211 |
| RAG2-EGFP | 106 ± 10.3 (59.2 ± 5.7) | 40.7 ± 5.2 (22.6 ± 2.8) | 15.8 ± 5.5 (8.8 ± 3.0) | 7.5 ± 4.3 (4.3 ± 2.4) | 229 ± 28 | 1,362 |
Total cell numbers and mean percentage of cells corresponding to erythroid, myeloid, lymphoid, and progenitor compartments were analyzed by FACS. Clonable B cell in 103 kidney marrow progenitors of wild-type and TEL-AML1 transgenic fish, and in the EGFP-positive (lymphoid progenitor enriched) fraction of marrow progenitors from RAG2-EGFP-TA and RAG2-EGFP control fish. Data are presented as the mean ± SD of 12 replicates from each transgenic line done in two independent experiments.
*Significantly higher number of progenitors or lower number of colonies, compared to wild-type or control cells.
Infiltrating Leukemia in TEL-AML1 Transgenic Zebrafish.
The expression of TEL-AML1 or EGFP-TEL-AML1 from the ubiquitous XEF and ZBA promoters, which drive TEL-AML1 expression in all lineages, including noncommitted progenitors, led to the development of lymphoblastic leukemia in ≈3% (16 of 545 = 2.93%) of transgenic fish. Leukemia developed with a latency of 8–12 months. None of the 353 RAG2-drivenTEL-AML1 fish, 125 ZBA- and RAG2-EGFP fish, or any of several hundred wild-type fish developed leukemias during the 36-month observation period.
Transgenic fish that developed leukemia were either found dead, or became moribund with progressive pallor and cachexia and were killed. Blood smears revealed a total leukocyte count of 38,000–52,000 cells per μl. Total red cell counts ranged from 0.2 to 3 × 106 cells per μl. The absolute blood lymphoblastic count was between 34,860 and 50,960 cells per μl, with 92–98% blasts.
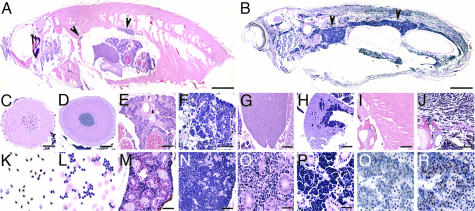
Histological sections confirmed the presence of lymphoblastic leukemia. A representative F1 transgenic fish from the XEF-EGFP-TA line (Fig. 3B) demonstrates dense deposits of small lymphoid-like blasts. The enlarged head and tail kidney (Fig. 3 B, N, and P) compared with wild-type (Fig. 3 A, M, and O), and the anatomical pattern (Fig. 3B) suggests that leukemia originated in the kidney. Compared with wild-type sections (Fig. 3 C, E, G, I, K, M, and O), leukemic cells disseminated into distant organs including the brain (Fig. 3D), ovary (Fig. 3F), liver (Fig. 3H), muscle (Fig. 3J), and completely replaced the kidney marrow (Fig. 3 N and P). Peripheral blood smears demonstrated clusters of small basophilic round neoplastic cells with open chromatin pattern and only a rim of dark cytoplasm (Fig. 3L). These cells were negative for myeloperoxidase (MPO) and periodic acid Schiff (PAS) staining (data not shown), indicating that they are neither myeloid nor erythroleukemic cells. Anti-EGFP immunohistochemistry confirmed the EGFP expression in these leukemic cells (Fig. 3 Q and R).
Fig. 3.
Leukemic features of TEL-AML1 transgenic zebrafish. (A and B) H&E stain of sagittal sections from wild-type (A) and an F1 XEF-EGFP-TA transgenic zebrafish (B), with diffuse infiltrates of basophilic blast-like cells, most dense in the kidney region (arrows indicate head and tail kidney). Leukemic cells infiltrated distal organs including the brain (D), ovary (F), liver (H), and muscle (J), compared with wild-type sections from these organs (C, E, G, and I). Peripheral blood smear from wild-type fish (K) showing normal nucleated RBCs, lymphocytes, and a monocyte, whereas a leukemic blood smear (L) shows clusters of lymphoblasts. The kidney section revealed complete infiltration of the marrow between the tubules shown in low (N) and high (P) power, compared with wild-type (M and O). (Q and R) Leukemic cells express EGFP-TEL-AML1. (Q) No staining without the primary anti-EGFP antibody. (R) TEL-AML1 lymphoblasts showed strong nuclear and cytoplasmic staining with anti-EGFP antibody (arrows). (Scale bars, 3 mm in A and B; 100 μm in C–M and O; and 50 μm in N, P and Q and R.)
Transplantation of TEL-AML1-Induced Leukemia.
To investigate whether leukemic cells from TEL-AML1 transgenic fish would multiply and propagate the disease to irradiated recipients, we isolated kidney marrow leukemic cells, constituting 96% of the marrow, from an XEF-TA leukemic fish. Injection of 5 × 105 leukemic cells IP into three wild-type recipients 2 days after conditioning with 25 Gy generated lethal infiltrating leukemias in recipients. Recipient fish displayed signs of disease between 6 and 9 weeks after transplant, with evidence of infiltrating leukemias with lymphoblasts morphologically indistinguishable form the primary donor leukemia (Fig. 9, which is published as supporting information on the PNAS web site).
TEL-AML1-Induced Leukemias Mimic Childhood CD10+ pre-B ALL.
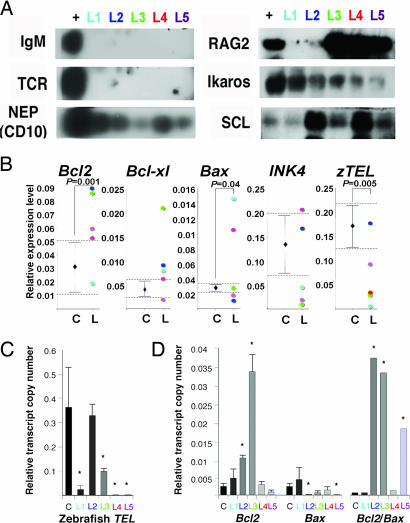
To identify the origin of TEL-AML1-induced leukemia in transgenic zebrafish, we subjected five leukemia samples to RT-PCR, followed by Southern blotting with zebrafish probes against the constant regions of IgM and TCR-α, the orthologue of human neutral endopeptidase (NEP) (CD10), RAG2, Ikaros, and SCL (TAL1) (Fig. 4A) (Supporting Text, which is published as supporting information on the PNAS web site). The leukemic cells were negative for IgM and TCR-α and expressed the zebrafish NEP (Fig. 4A). Four of five leukemias expressed RAG2, and all five were positive for the lymphoid progenitor marker, Ikaros, and the stem cell marker, SCL (Fig. 4A), confirming the lymphoid nature of TEL-AML1-induced leukemia. Southern blotting of genomic DNA, treated with the restriction enzyme BglII, from five leukemias and hybridization with probes against IgM and TCR-α, showed an oligoclonal pattern compared with wild-type (data not shown).
Fig. 4.
Molecular analysis of TEL-AML1 induced leukemia in transgenic zebrafish. (A) Southern blotting of RT-PCR products from five leukemia samples and positive control. (B) Semiquantitative RT-PCR showing the relative expression of each regulatory gene expressed as a ratio to β-actin to normalize the number of leukemic blasts. First, the expression range (dotted lines) in kidney marrow cells from wild-type and nonleukemic TEL-AML1 transgenic fish was established (C, control; n = 15). The expression levels from each of the five TEL-AML1 leukemic zebrafish are depicted (L, leukemias n = 5). (C) Down-regulation of zebrafish TEL transcripts in TEL-AML1 induced leukemia measured by quantitative one-step real-time RT-PCR. The value is presented as a ratio normalized to β-actin when a ratio of 1 represents the normalized expression ratio in wild-type. (D) Apoptotic signal changes in leukemic fish displayed as the mean (± SD) transcript copy number relative to β-actin. A survival determinant high Bcl2/Bax ratio was 7- to 8-fold higher than control in L2 (∗, P = 0.004), L3 (∗, P = 0.0043), and L5 (∗, P = 0.0063). Data represent three independent experiments done in triplicates.
Gene Expression Signature of TEL-AML1-Induced Zebrafish ALL.
The low frequency and long latency before leukemia development in transgenic zebrafish suggested that secondary mutations were necessary for transformation. To investigate the variations that confer a proliferative or survival advantage (30) to TEL-AML1-expressing progenitors, we compared tumor suppressor, cell cycle, and apoptotic gene expression of TEL-AML1-induced ALL cells (n = 5) with those of both nonleukemic TEL-AML1-expressing marrow cells (n = 10), and wild-type marrow cells (n = 5) (Fig. 4 B–D). RT-PCR analysis of TEL-AML1-associated leukemias showed significant down-regulation of the endogenous zebrafish TEL, and deregulation of zebrafish apoptotic Bcl2, Bcl-xl, and Bax (Fig. 4B). Down-regulation of zebrafish TEL in four leukemias was confirmed by analyzing transcript copy numbers using one-step quantitative real-time RT-PCR (Fig. 4C). Additionally, apoptotic molecular determinants favoring cell survival with high Bcl2/Bax ratio were present in three TEL-AML1 leukemias with overexpression of Bcl2 in two leukemias, and down-regulation of Bax in a third leukemia (Fig. 4D).
Although the two groups of leukemic and nonleukemic TEL-AML1 cells were not found to be statistically significant in expression of the zebrafish orthologue of INK4 (P16) (Fig. 4B), the zebrafish tumor suppressors tp53, MDM2, pRB, and the cell cycle check point P21 and P27 (Kip1) pathways (Fig. 10, which is published as supporting information on the PNAS web site), individual expression profiles point to mutations in cell cycle regulatory pathways. Three of the five leukemias showed down-regulation of INK4, and two of the three were associated with pRB down-regulation (Fig. 10).
Discussion
Despite the well described prevalence of the TEL-AML1 fusion in pre B cell ALL, the leukemic cell of origin and the molecular pathway of transformation have not been identified. These studies describing the development of a TEL-AML1 transgenic zebrafish identify the cellular targets for TEL-AML1, and elucidate the potential pathways to transformation.
Twin studies pioneered by Greaves have provided the strongest evidence that childhood leukemia is initiated before birth (5). Epidemiologic studies (31) and mathematical modeling also support the fetal initiation of ALL (32). The TEL-AML1 fusion was detected in 1% of cord blood samples (4), a frequency 1,000-fold higher than ALL incidence in children, suggesting that secondary genetic events are involved in transformation.
In the TEL-AML1 transgenic mouse, the expression of TEL-AML1 was driven by the Eμ promoter, active in B-lymphocytes from the pre-B cell stage to the mature B cells. These mice did not develop any hematological disorder (7). The observation that none of the RAG2 zebrafish expressing TEL-AML1 in lymphoid progenitors developed leukemia or progenitor expansion points to a noncommitted progenitor origin of TEL-AML1 leukemia. RAG expression, undetectable in mature mouse T and B lymphocytes or HSC (33), is first detected in the lineage committed AA4.1+ HSA− B220+ CD4+ CD43+ (Hardy fraction A1) pro-B cells (34). The same fraction was shown to expand in response to TEL-AML1 expression in the mouse transplant model (9). Together, these data indicate that TEL-AML1, when expressed in noncommitted progenitors, exerts its differentiation blockade at the transition from pro-B to pre-B cells. Although there remains the possibility that B cell maturation in zebrafish is not identical to mammals, this appears to be an unlikely explanation.
Both TEL and AML1 are commonly involved in chromosomal rearrangements (1, 35, 36). AML1 is a master regulator of definitive hematopoiesis (30, 37). TEL-AML1 may exert dominant-interfering effects on TEL-induced transcriptional repression (28, 38), and inhibit AML1 transcriptional activity (37), altering both self-renewal and differentiation of HSC (30). These effects on HSC differentiation would explain the expanded progenitors and the B cell differentiation deficit observed in both TEL-AML1 transgenic zebrafish and mouse transplant models (8, 9), and establish TEL-AML1 as class II mutation impairing differentiation (30).
Distinct expression profiles characterize TEL-AML1 associated human ALL (39). We examined several genes comprising the expression signature in TEL-AML1 transgenic fish to determine whether alterations in the tumor suppressor, apoptotic, or cell cycle signals were associated with leukemic transformation. The endogenous zebrafish TEL (also known as ETV6) was down-regulated when leukemia developed. TEL is a putative tumor suppressor gene based on the frequent loss of the normal TEL allele or loss of heterozygosity (LOH) in leukemia, which may represent a secondary event essential for transformation (3, 40). Additionally, our data demonstrate a deregulated expression of the apoptotic genes Bcl2, BAX, and Bcl-xl, which Inhibit apoptosis or promote cell cycle arrest in HSC. Patients with TEL-AML1-positive ALL displayed a unique expression pattern of 16 key apoptosis genes, including Bcl2 family members (41). This Bcl-2 differential expression may induce an apoptotic blockage that permits survival and selection of aggressive clones during tumor clonal evolution. Moreover, AML1-ETO fusion, another class II mutation associated with AML, activates Bcl-2 transcription by binding to the Bcl-2 promoter (42). Therefore, apoptotic defects, generated by the differential expression of Bcl2 genes, when coupled with deregulated proliferation (43) induced by class II mutations, may trigger leukemic development.
The Ink4 locus in mammals encodes three proteins that modulate the pRB and tp53 tumor suppressor pathways (44). Loss of the Ink4a was shown to cooperate with TEL-AML1 to induce leukemia in mice (11); however, long latency was still required. Similarly, three of five zebrafish leukemias analyzed expressed lower levels of the zebrafish Ink4 orthologue. It was predicted that, unlike mammals, the tp53 and pRB pathways are not regulated by a single locus in puffer fish, Fugu rubripes (45). We found no genomic evidence of the presence of a p19-like ARF-encoding potential in the zebrafish Ink4 locus. In three leukemic fish analyzed, pRB levels were lower than controls, revealing a defect in the p16Ink4a–pRB pathway.
TEL-AML1-induced leukemias were negative for TCR-α and IgM, expressed the conserved CD10 domain, and the lymphoid transcription factor Ikaros, a characteristic feature of human CD10+ ALL (46). Of note, all TEL-AML1-induced leukemias expressed the stem cell marker SCL, and four of the five leukemias expressed RAG2, despite the fact that directing TEL-AML1 to the RAG2-expressing progenitors failed to induce leukemia.
Our data support a multistep model for TEL-AML1-associated leukemia where TEL-AML1 expression in noncommitted progenitors, before the common lymphoid progenitor, generates multiple long-lived preleukemic clones likely to arrest at the pre-B cell differentiation stage. Leukemia develops when these preleukemic clones acquire the capacity for indefinite self-renewing proliferation by accumulation of mutations and/or epigenetic changes that, at least in part, increase proliferation and/or block apoptosis of the leukemic stem cell clone(s).
The identification of possible leukemic pathways in the TEL-AML1 transgenic zebrafish may further our understanding of the leukemic process, and potentially lead to the identification of new therapeutic targets.
Methods
Zebrafish Maintenance.
Wild-type EK and AB* zebrafish (Danio rerio) were maintained in the National Cancer Institute animal facility following National Institutes of Health guidelines for care of small aquatic animals. Adult fish were spawned and reared in conditioned water at 28.5°C on a 14-h light/10-h dark cycle. Embryos obtained by spontaneous spawning were collected and staged as described in the online zebrafish information database (http://zfin.org) (47).
Generation of TEL-AML1 Transgenic Zebrafish.
Five cDNA constructs were generated (Fig. 1A) using the highly conserved human TEL-AML1 fusion cDNA encompassing the first five exons of TEL (encoding residues 1–133) and the full-length splice variant of AML1 (AML1-c) exons 2–8 (encoding amino acids 21–479) (35). TEL-AML1 fusion was generated by PCR from Reh cell template based on sequences from TEL-AML1 patients (35). In the EGFP fusion constructs, TEL-AML1 was fused in-frame to the C terminus of EGFP (Supporting Text). For ubiquitous expression, a 0.7-kb fragment of Xenopus EF1α promoter (24) and a 4.5-kb fragment (H.-J.T., unpublished data) modified from the zebrafish β-actin promoter (23) were used. The 6.5-kb zebrafish Rag2 promoter (25) was used to target TEL-AML1 expression to lymphoid progenitors. The TEL-AML1-containing vectors were linearized immediately 5′ to the promoter sequences, and each were microinjected at a concentration of 20–100 ng/μl into one- to two-cell stage zebrafish embryos. The injected fish were selected by the uniform dye distribution for TEL-AML1 constructs or EGFP expression for EGFP-TEL-AML1 constructs, and were grown to maturity. Potential founders were bred with wild-type fish, and embryos expressing EGFP were selected at 24 h postfertilization for the ZBA-EGFP-TA line, and in the thymic cells at 7 dpf in RAG2-EGFP-TA line, whereas in lines without EGFP, transgenic founders and progeny were identified by detecting TEL-AML1 by PCR (Supporting Text).
RT-PCR Analysis of TEL-AML1 Transcripts.
Fertilized eggs from the breeding of a positive male with a wild-type female were collected, and total RNA from 10–20 embryos was extracted. In both the RAG2-TA and RAG2-EGFP-TA lines, adult fish were anesthetized with tricaine methane sulfonate (MS 222, Argent Laboratories, Redmond, WA), the heart, muscle, kidney, and thymus were separated and immediately frozen in liquid nitrogen. Total RNA from embryos or adult tissue was used as template for One-step RT-PCR (Invitrogen, Carlsbad, CA) (Supporting Text). RT-PCR conditions were as follows: 42°C for 50 min, 95°C for 5 min, 35 cycles of 94°C for 30 s, 60°C for 30 s, 72°C for 1 min, then 72°C for 10 min.
FACS Analysis.
Kidney marrow progenitors were determined by flow cytometry (FACSCaliber, BD Immune-Cytometry, San Jose, CA), and were collected, whereas dead cells, erythrocytes, granulocytes, and lymphocytes were excluded based on propidium iodide or DRAQ5 uptake, forward angle light scatter, or 90° side scatter, as described (27). Briefly, kidney marrow cells from adult male wild-type, control EGFP, or TEL-AML1 transgenic fish were resuspended in ice-cold 0.9% PBS with 10% FBS and passed through a 40-μm filter. Cells were washed in ice-cold 0.9% PBS with 10% FBS and stained with either 5 μM DRAQ5 (Biostatus, Leicestershire, U.K.) or 1 μg/ml propidium iodide (Sigma, St. Louis, MO) to exclude dead cells, and progenitor fractions were sorted and used for the clonogenic assays.
Mitogen-Induced B Cell Colony-Forming Assays.
FACS analysis was based on forward and side scatter (27) and on EGFP expression from the ZBA-EGFP-TA and the RAG2-EGFP-TA transgenic fish compared with wild-type and ZBA-EGFP control fish. Cells were prestimulated for 24 h in Dulbecco's Modification of Eagle's Medium/Ham's F-12 1/1 Mix (DMEM/F12) containing 15% heat inactivated carp serum (SeaGrow, Eastcoast Bio, North Berwick, ME), and then plated in 12 replicates at 5 × 104 cells in Bacto agar (BD Biosciences, San Jose, CA) under B cell differentiation conditions (29). Briefly, cells were cultured in 0.3% Bacto agar in DMEM/F12 containing 7.5% carp serum, 7.5% FBS, 50 μM 2-mercaptoethanol, 100 μM MEM nonessential amino acids, with 292 μg/ml l-glutamine and 1 mM sodium pyruvate, 1% penicillin and streptomycin, and supplemented with 25 μg/ml LPS from Salmonella typhi (Sigma). Cells were plated in 0.5-ml volume in 24-well plates and cultured in a humidified incubator at 28°C, 5% CO2 in air. Colonies containing 50 cells or more were counted at days 6–8, plates were fixed and stained with May–Grunwald/Giemsa.
Southern Analysis and RT-PCR of Leukemic Cells.
Leukemic cells were separated from the kidney marrow or by cardiac puncture. Genomic DNA was digested with BglII, whereas RNA was extracted by using TRIzol (Invitrogen) and subjected to one-step RT-PCR or cDNA synthesis (Supporting Text). The resolved DNA or RT-PCR products were transferred to nylon membranes and hybridized with zebrafish probes against Tcr-α and IgM constant regions, RAG2, Ikaros, SCL, and the neutral endopeptidase (NEP) (CD10) conserved zebrafish domain. All probes were amplified by PCR from 1- or 7-dpf zebrafish cDNA library, cloned in TOPO vectors (Supporting Text), then labeled with alkaline phosphatase by using the universal linkage system (Amersham Pharmacia, Piscataway, NJ). One-step quantitative real-time RT-PCR analyses were done by using the Lightcycler (Roche, Basel, Switzerland) as described (48) starting with a reverse transcription cycle of 48°C for 30 min.
Leukemic Cell Transplantation into Irradiated Wild-Type Adults.
A leukemic 12-month-old transgenic F2 XEF-TA fish was killed, and leukemic cells were transplanted to wild-type recipients as described (27). Briefly, three recipient fish were anesthetized, sublethally irradiated with 25 GY (from a 137Cs source), and injected i.p. 2 days after irradiation with 5 × 105 total kidney cells. Diseased fish were killed, and blood smears and histological analysis showed evidence of leukemia.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Abbreviations
- ALL
acute lymphoblastic leukemia
- dpf
days postfertilization.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pui CH, Relling MV, Downing JR. N Engl J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 2.Rowley JD. Semin Hematol. 1999;36:59–72. [PubMed] [Google Scholar]
- 3.Romana SP, Poirel H, Leconiat M, Flexor MA, Mauchauffe M, Jonveaux P, Macintyre EA, Berger R, Bernard OA. Blood. 1995;86:4263–4269. [PubMed] [Google Scholar]
- 4.Mori H, Colman SM, Xiao Z, Ford AM, Healy LE, Donaldson C, Hows JM, Navarrete C, Greaves M. Proc Natl Acad Sci USA. 2002;99:8242–8247. doi: 10.1073/pnas.112218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiemels JL, Ford AM, Van Wering ER, Postma A, Greaves M. Blood. 1999;94:1057–1062. [PubMed] [Google Scholar]
- 6.Teuffel O, Betts DR, Dettling M, Schaub R, Schafer BW, Niggli FK. Leukemia. 2004;18:1624–1629. doi: 10.1038/sj.leu.2403462. [DOI] [PubMed] [Google Scholar]
- 7.Andreasson P, Schwaller J, Anastasiadou E, Aster J, Gilliland DG. Cancer Genet Cytogenet. 2001;130:93–104. doi: 10.1016/s0165-4608(01)00518-0. [DOI] [PubMed] [Google Scholar]
- 8.Morrow M, Horton S, Kioussis D, Brady HJ, Williams O. Blood. 2004;103:3890–3896. doi: 10.1182/blood-2003-10-3695. [DOI] [PubMed] [Google Scholar]
- 9.Tsuzuki S, Seto M, Greaves M, Enver T. Proc Natl Acad Sci USA. 2004;101:8443–8448. doi: 10.1073/pnas.0402063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer M, Schwieger M, Horn S, Niebuhr B, Ford A, Roscher S, Bergholz U, Greaves M, Lohler J, Stocking C. Oncogene. 2005;24:7579–7591. doi: 10.1038/sj.onc.1208931. [DOI] [PubMed] [Google Scholar]
- 11.Bernardin F, Yang Y, Cleaves R, Zahurak M, Cheng L, Civin CI, Friedman AD. Cancer Res. 2002;62:3904–3908. [PubMed] [Google Scholar]
- 12.Pine SR, Wiemels JL, Jayabose S, Sandoval C. Leuk Res. 2003;27:155–164. doi: 10.1016/s0145-2126(02)00183-2. [DOI] [PubMed] [Google Scholar]
- 13.Hotfilder M, Rottgers S, Rosemann A, Jurgens H, Harbott J, Vormoor J. Blood. 2002;100:640–646. doi: 10.1182/blood.v100.2.640. [DOI] [PubMed] [Google Scholar]
- 14.Bennett CM, Kanki JP, Rhodes J, Liu TX, Paw BH, Kieran MW, Langenau DM, Delahaye-Brown A, Zon LI, Fleming MD, Look AT. Blood. 2001;98:643–651. doi: 10.1182/blood.v98.3.643. [DOI] [PubMed] [Google Scholar]
- 15.Zon LI. Blood. 1995;86:2876–2891. [PubMed] [Google Scholar]
- 16.Stanton MF. J Natl Cancer Inst. 1965;34:117–130. doi: 10.1093/jnci/34.1.117. [DOI] [PubMed] [Google Scholar]
- 17.Kalev-Zylinska ML, Horsfield JA, Flores MV, Postlethwait JH, Vitas MR, Baas AM, Crosier PS, Crosier KE. Development (Cambridge, UK) 2002;129:2015–2030. doi: 10.1242/dev.129.8.2015. [DOI] [PubMed] [Google Scholar]
- 18.Langenau DM, Traver D, Ferrando AA, Kutok JL, Aster JC, Kanki JP, Lin S, Prochownik E, Trede NS, Zon LI, Look AT. Science. 2003;299:887–890. doi: 10.1126/science.1080280. [DOI] [PubMed] [Google Scholar]
- 19.Langenau DM, Feng H, Berghmans S, Kanki JP, Kutok JL, Look AT. Proc Natl Acad Sci USA. 2005;102:6068–6073. doi: 10.1073/pnas.0408708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onnebo SM, Condron MM, McPhee DO, Lieschke GJ, Ward AC. Exp Hematol. 2005;33:182–188. doi: 10.1016/j.exphem.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Montpetit A, Sinnett D. Oncogene. 2001;20:3437–3442. doi: 10.1038/sj.onc.1204444. [DOI] [PubMed] [Google Scholar]
- 22.Burns CE, DeBlasio T, Zhou Y, Zhang J, Zon L, Nimer SD. Exp Hematol. 2002;30:1381–1389. doi: 10.1016/s0301-472x(02)00955-4. [DOI] [PubMed] [Google Scholar]
- 23.Higashijima S, Okamoto H, Ueno N, Hotta Y, Eguchi G. Dev Biol. 1997;192:289–299. doi: 10.1006/dbio.1997.8779. [DOI] [PubMed] [Google Scholar]
- 24.Johnson AD, Krieg PA. Gene. 1994;147:223–226. doi: 10.1016/0378-1119(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 25.Jessen JR, Jessen TN, Vogel SS, Lin S. Genesis. 2001;29:156–162. doi: 10.1002/gene.1019. [DOI] [PubMed] [Google Scholar]
- 26.Trede NS, Zapata A, Zon LI. Trends Immunol. 2001;22:302–307. doi: 10.1016/s1471-4906(01)01939-1. [DOI] [PubMed] [Google Scholar]
- 27.Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, Zon LI. Nat Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 28.Kwiatkowski BA, Bastian LS, Bauer TR, Jr, Tsai S, Zielinska-Kwiatkowska AG, Hickstein DD. J Biol Chem. 1998;273:17525–17530. doi: 10.1074/jbc.273.28.17525. [DOI] [PubMed] [Google Scholar]
- 29.Kincade PW, Ralph P, Moore MA. J Exp Med. 1976;143:1265–1270. doi: 10.1084/jem.143.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speck NA, Gilliland DG. Nat Rev Cancer. 2002;2:502–513. doi: 10.1038/nrc840. [DOI] [PubMed] [Google Scholar]
- 31.Ross JA, Davies SM, Potter JD, Robison LL. Epidemiol Rev. 1994;16:243–272. doi: 10.1093/oxfordjournals.epirev.a036153. [DOI] [PubMed] [Google Scholar]
- 32.Smith MA, Chen T, Simon R. J Natl Cancer Inst. 1997;89:1542–1544. doi: 10.1093/jnci/89.20.1542. [DOI] [PubMed] [Google Scholar]
- 33.Nagaoka H, Yu W, Nussenzweig MC. Curr Opin Immunol. 2000;12:187–190. doi: 10.1016/s0952-7915(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 34.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golub TR, Barker GF, Bohlander SK, Hiebert SW, Ward DC, Bray-Ward P, Morgan E, Raimondi SC, Rowley JD, Gilliland DG. Proc Natl Acad Sci USA. 1995;92:4917–4921. doi: 10.1073/pnas.92.11.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odero MD, Carlson K, Calasanz MJ, Lahortiga I, Chinwalla V, Rowley JD. Genes Chromosomes Cancer. 2001;31:134–142. doi: 10.1002/gcc.1127. [DOI] [PubMed] [Google Scholar]
- 37.Lutterbach B, Hiebert SW. Gene. 2000;245:223–235. doi: 10.1016/s0378-1119(00)00014-7. [DOI] [PubMed] [Google Scholar]
- 38.Gunji H, Waga K, Nakamura F, Maki K, Sasaki K, Nakamura Y, Mitani K. Biochem Biophys Res Commun. 2004;322:623–630. doi: 10.1016/j.bbrc.2004.07.169. [DOI] [PubMed] [Google Scholar]
- 39.Ross ME, Zhou X, Song G, Shurtleff SA, Girtman K, Williams WK, Liu HC, Mahfouz R, Raimondi SC, Lenny N, Patel A, Downing JR. Blood. 2003;102:2951–2959. doi: 10.1182/blood-2003-01-0338. [DOI] [PubMed] [Google Scholar]
- 40.Patel N, Goff LK, Clark T, Ford AM, Foot N, Lillington D, Hing S, Pritchard-Jones K, Jones LK, Saha V. Br J Haematol. 2003;122:94–98. doi: 10.1046/j.1365-2141.2003.04399.x. [DOI] [PubMed] [Google Scholar]
- 41.Holleman A, den Boer ML, de Menezes RX, Cheok MH, Cheng C, Kazemier KM, Janka-Schaub GE, Gobel U, Graubner UB, Evans WE, Pieters R. Blood. 2006;107:769–776. doi: 10.1182/blood-2005-07-2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klampfer L, Zhang J, Zelenetz AO, Uchida H, Nimer SD. Proc Natl Acad Sci USA. 1996;93:14059–14064. doi: 10.1073/pnas.93.24.14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green DR, Evan GI. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 44.Sherr CJ. Nat Rev Mol Cell Biol. 2001;2:731–737. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]
- 45.Gilley J, Fried M. Oncogene. 2001;20:7447–7452. doi: 10.1038/sj.onc.1204933. [DOI] [PubMed] [Google Scholar]
- 46.Nishii K, Katayama N, Miwa H, Shikami M, Usui E, Masuya M, Araki H, Lorenzo F, Ogawa T, Kyo T, Nasu K, Shiku H, Kita K. Leukemia. 2002;16:1285–1292. doi: 10.1038/sj.leu.2402533. [DOI] [PubMed] [Google Scholar]
- 47.Westerfield M, Doerry E, Kirkpatrick AE, Driever W, Douglas SA. Semin Cell Dev Biol. 1997;8:477–488. doi: 10.1006/scdb.1997.0173. [DOI] [PubMed] [Google Scholar]
- 48.Pine SR, Yin C, Matloub YH, Sabaawy HE, Sandoval C, Levendoglu-Tugal O, Ozkaynak MF, Jayabose S. J Mol Diagn. 2005;7:127–132. doi: 10.1016/S1525-1578(10)60018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.