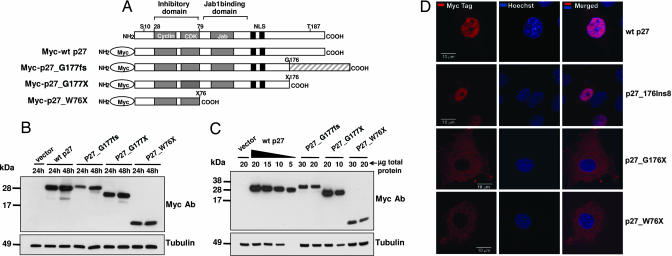
Fig. 4.
Expression and subcellular localization of exogenous proteins during transfection. (A) Schematic representation of Myc-tagged p27 proteins used for transfections. The shaded area at the C terminus of p27_1020;G177fs represents the p27-unrelated amino acids present in the MENX mutant protein. (B and C) Immunoblots showing the expression of the transfected proteins. (B) MCF-7 cells were transfected with 1 μg of the indicated constructs. Cell lysates were prepared at the indicated times after transfection; 50 μg of total protein was separated by electrophoresis, blotted, and probed with antibodies to the Myc tag. To control for equal loading, the membrane was probed with the anti-tubulin monoclonal antibody. (C) MCF-7 cells were transfected as in B. Twenty-four hours later, cells were harvested, and proteins were extracted. Different amounts of total protein lysates (indicated) were loaded onto the gel. p27_1020;G177fs and of p27_1020;W76X proteins seem to be ≈6-fold less abundant than the WT p27 protein. (D) Subcellular localization of Myc-tagged p27 proteins. Rat2 cells were transfected with the Myc-WTp27, Myc-p27_1020;G177fs, Myc-p27_1020;G177X, and p27_1020;W76X (indicated on the right) constructs. Forty-eight hours later, the transfected cells were fixed and permeabilized, and then they were stained with an antibody to the Myc tag visualized with Cy3 (red). Nuclei were counterstained with Hoechst (blue). (Left) Cy3. (Center) Hoechst. (Right) Merge. (Scale bars: 10 μm.)