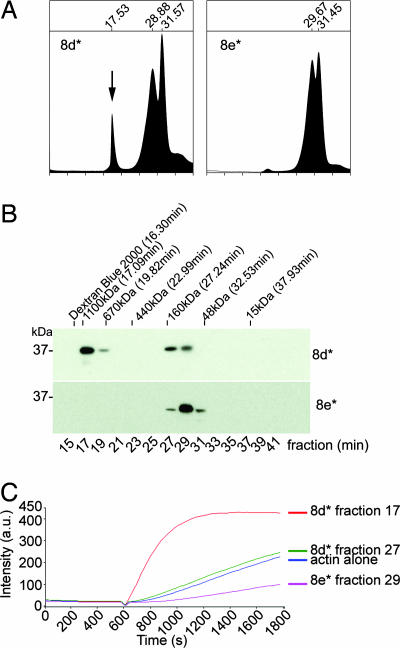
Fig. 5.
The actin-binding domain of TARP oligomerizes to form an actin nucleator. TARP fragments 8d* (* indicates GST is removed) capable of polymerizing actin and 8e* missing a proline-rich domain were analyzed by gel filtration. (A) An additional peak representing oligomerized Tarp (arrow) was detected in the A280 trace of eluted fragment 8d*. Elution times are indicated above the major peaks. The protease used to remove the GST moiety eluted at the 31.5-min peak. (B) Protein fractions were collected in 2-min intervals from the gel filtration column. Protein fractions were resolved by SDS/PAGE and were subjected to immunoblotting with a TARP-specific antibody. Dextran Blue 2000, polyacrylic sizing beads, and protein standards are indicated above the immunoblot with respective molecular weight and peak elution times. (C) Oligomerized TARP peptide polymerizes pyrene actin. GST-TARP peptide 8d* fraction 17 increased actin polymerization compared with the smaller 8d* fraction 27, which polymerized actin to similar levels as the actin alone control. 8e* fraction 29 reduced the rate of actin polymerization.