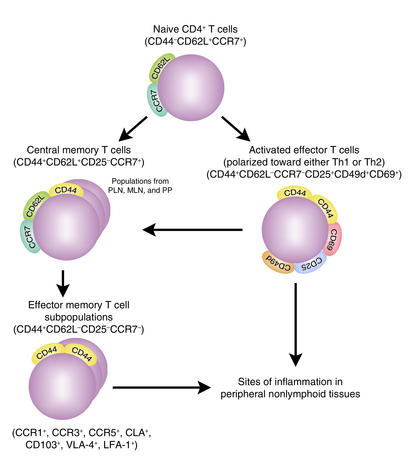
Figure 2.
Stepwise differentiation of CD4+ T cells from naive CD4+ T cells to activated effector CD4+ T cells, and then to subsets of memory CD4+ T cells. These are divided into central memory CD4+ T cells (CD44+CD62L+CD25–CCR7+) and effector memory CD4+ T cells (CD44+CD62L–CD25–CCR7–). CD44+CD62L–CD25–CCR7–CD4+ effector memory T cells represent a pool of terminally differentiated cells with heterogeneous immunophenotypes that express multiple patterns of chemokine receptors (i.e., CCR1, 3, or 5), tissue-specific homing receptors (i.e., CD103 and the cutaneous lymphocyte antigen [CLA]), and adhesion molecules (i.e., leukocyte function–associated antigen-1 [LFA-1] and very late antigen-4 integrin [VLA-4]), facilitating migration of these cells into different peripheral nonlymphoid tissues. The unique expression pattern and level of chemokine receptors, homing receptors, and adhesion molecules depend on the lymphoid origin (i.e., peripheral lymph nodes [PLNs], mesenteric lymph nodes [MLNs], or Peyer’s patches [PPs]) of the effector memory T cells and the duration of TCR and cytokine stimulation. These features determine the migratory and homing preferences and functional capabilities of CD44+CD62L–CD25–CCR7–CD4+ effector memory T cells.