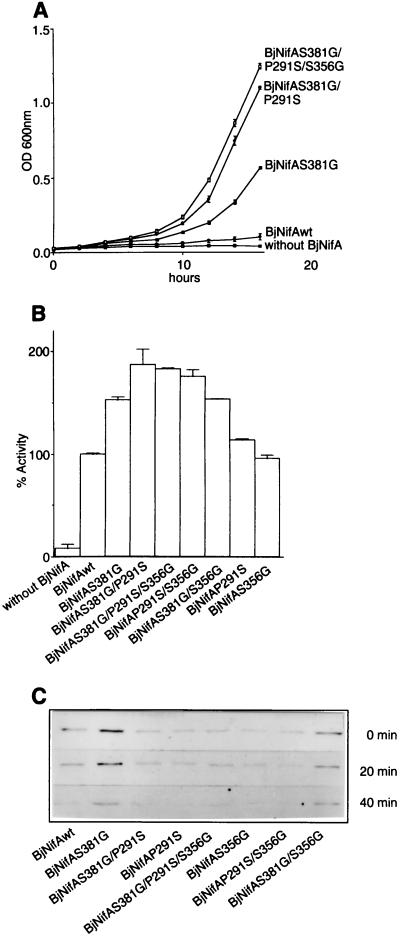
Figure 3.
Transcriptional activity, stability, and sensitivity to oxygen of the BjNifA derivatives. (A) Growth curves of strains carrying the wild type or the directly evolved BjNifA derivatives, as indicated. Cells were grown on NFDM medium with Cm (40 μg/ml) under aerobic conditions. The level of expression of the cat gene in pVB007, under the S. meliloti nifH promoter (deleted of the enhancer), and, therefore, the resistance to Cm is dependent on the activity of each BjNifA derivative. Values represent the mean ± SD of three independent experiments. (B) Transcriptional activation of a nifH-lacZ fusion by the BjNifA mutant derivatives. (C) Stability and sensitivity of BjNifA mutant proteins to oxygen. To analyze whether the mutations affected the stability of the protein or any intrinsic function leading to a higher transcriptional activation, we grew strains carrying each of the mutant BjNifA proteins in microaerobic cultures, and the amount of the protein was detected after being shifted to heavily aerated flasks. Samples were taken at time 0 min, 20 min, and 40 min, and the BjNifA derivatives were immunodetected in soluble cell extracts with an antibody raised against a polypeptide comprising part of the central domain. All mutant proteins had similar stability and oxygen sensitivity because they decreased at the same rate as the wild type after being shifted to air.