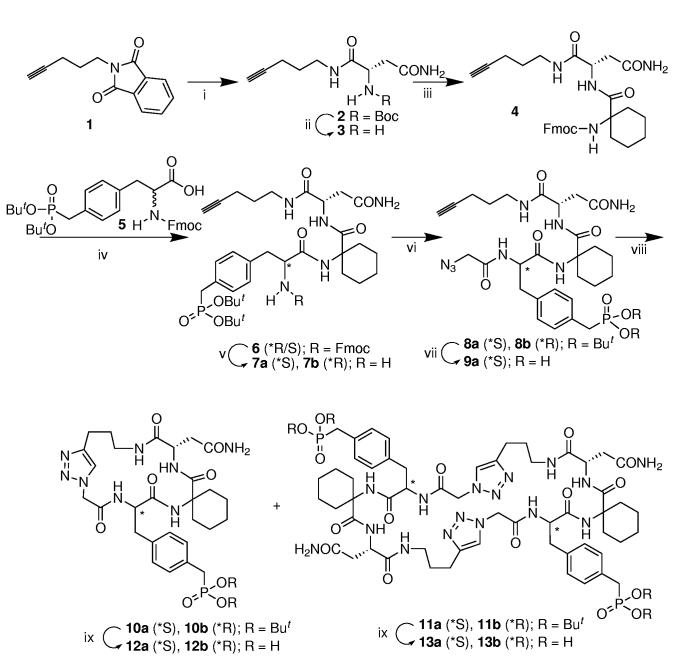
Scheme 1.
(i) a) N2H4•H2O, EtOH, H2O, reflux, 2 h; b) Boc-Asn-OH, EDC, HOBt, DMF, rt, 12 h, (48% yield for two steps); (ii) a) TFA, CH2Cl2, rt, 1 h (95% yield); (iii) a) Fmoc-1-amino-cyclohexanecarboxylic acid, EDC, HOBt, DMF, rt, 12 h (84% yield); (iv) a) piperidine, CH3CN, rt, 2 h, b) EDCI•HCl, HOBt, DMF, rt, 12 h (39% yield for two steps); (v) piperidine, CH3CN, rt, 2 h (37% yield for 7a; 38% yield for 7b); (vi) a) (BrCH2CO)2O DMF, rt, 1h; b) NaN3, DMF, rt, 24 h (two steps, 81% yield for 8a, 62% yield for 8b); (vii) TFA-HS(CH2)2SH-H2O, rt, 1 h (69% yield); (viii) CuI, L-ascorbate, DIPEA, CH3CN/tBuOH/H2O, rt; (ix) TFA-HS(CH2)2SH-H2O, rt, 1 h.