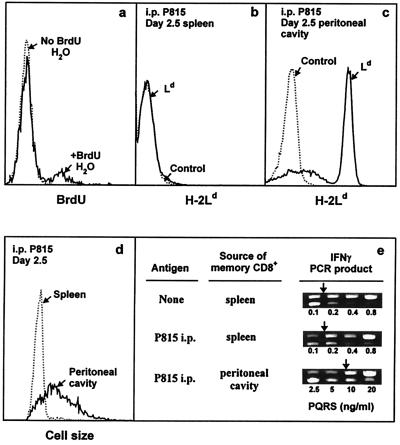
Figure 5.
In vivo activity of IL-4-induced long-term CD8+ memory T cells. (a) Each of two B10.TL hosts was transferred with 107 2C CD8+ T cells activated by B10.A B cell blasts + IL-4 as described in Materials and Methods. On day 88 postadoptive transfer, one host was given BrdUrd drinking water (1 mg/ml BrdUrd/2.5% glucose) and the other was given regular drinking water. On day 9, spleen cells were stained with Cy5-anti-Thy-1.2, followed sequentially by paraformaldehyde (0.5%) and acid (3 M HCl/0.5% Tween 20, 20 min) fixation, neutralization, F-anti-BrdUrd staining, and dual-parameter flow cytometric analysis. Histograms of BrdUrd incorporation by donor (Thy-1.2+) memory cells are shown. There were 12.1% BrdUrd+ cells among memory CD8+ T cells residing in hosts given BrdUrd drinking water (similar levels of BrdUrd labeling were observed for two similarly treated B10.TL hosts; data not shown). Negligible BrdUrd+ cells were found in the adoptive host given regular drinking water. (b– e) Each of two B10.TL mice was transferred with 3 × 106 Ld-reactive 2C CD8+ T cells activated by B10.A B cell blasts + IL-4. On day 277 postadoptive transfer, one host received an i.p. injection of 107 Ld-bearing P815 mastocytoma cells whereas the other did not. On day 2.5 post-P815 stimulation, cells recovered from the spleen and peritoneal cavity were analyzed. First, they were stained with F-anti-Ld to identify P815 cells in the spleen (b) and the peritoneal cavity (c). Second, they were stained with F-anti-Ld + Cy5-anti-Thy-1.2. Light-scatter histograms of donor (Thy-1.2+Ld-) cells from the spleen and peritoneal cavity are shown (d). Similarly identified donor spleen cells from the control host (no P815 challenge) had a nearly superimposable light-scatter distribution to the one shown for donor spleen cells from the P815-challenged host and was only marginally higher than the light-scatter distribution of freshly isolated naive (CD44lowCD62Lhi) CD8+ T cells (data not shown). Total recovered 2C donor cells from the P815-challenged mouse: spleen, 0.4 × 106; peritoneal cavity, 0.26 × 106. Total recovered 2C donor cells from P815-unchallenged mouse: spleen, 0.23 × 106; peritoneal cavity, not found. Third, they were stained as in c, and Thy-1.2+Ld-donor cells were isolated by cell sorting and analyzed for IFN-γ mRNA by nested competitive PCR (e). The concentrations of PQRS competitor DNA are as shown. The PCR products are: higher band, PQRS competitor; lower band, wild-type cDNA. Arrows mark equivalent amounts of wild-type and competitor PCR products.