Abstract
Proteolipid protein (PLP)-139–151 is the dominant encephalitogenic peptide that induces experimental autoimmune encephalomyelitis (EAE) in SJL (H-2s) mice. To examine the contribution of T cell receptor (TCR) specificity in the induction of EAE, we generated transgenic mice expressing the rearranged TCR genes from an encephalitogenic or a nonencephalitogenic PLP-139–151/I-As-specific T cell clone. Both types of transgenic lines developed spontaneous EAE, but, remarkably, the lines expressing the TCR from the nonencephalitogenic clone showed increasingly higher frequencies of disease (60–83%) in progressive SJL backcrosses and could not be propagated on the susceptible background. The T cells from the transgenic mice were not tolerized, because they responded vigorously to the antigen in vitro and mediated EAE when the mice were immunized with antigen. Besides being the only description of a TCR transgenic mice for the PLP-139–151/I-As epitope, the results demonstrate that the TCR from a nonencephalitogenic PLP-specific T cell clone can induce autoimmune disease when expressed appropriately in vivo.
Myelin proteolipid protein (PLP) is the major protein of the central nervous system (CNS) myelin. Multiple epitopes of PLP that can induce experimental autoimmune encephalomyelitis (EAE) in several different strains of mice have been identified (1–5). In SJL (H-2s) mice, there are two major encephalitogenic epitopes of PLP, PLP-139–151 and PLP-178–191 (2, 6), each of which binds with a binding affinity of K50 < 1 μM to I-As (5), but the immune response to PLP-139–151 is unusually dominant. First, immunization of SJL mice with whole spinal cord homogenate that contains multiple myelin antigens including myelin basic protein (MBP), PLP, and myelin oligodendrocyte glycoprotein results in a T cell response directed entirely to the PLP-139–151 epitope (7). Second, if PLP-139–151-specific T cells are tolerized in SJL mice, disease induction by whole spinal cord homogenate is abrogated (8). In addition to the unique features of this epitope, different H-2s strains show differences in their susceptibility to EAE. Whereas SJL mice are highly susceptible, B10.S mice are relatively resistant to the development of EAE when they are immunized with PLP-139–151. Thus, these mouse strains provide an opportunity to identify the genetic loci and genes that are responsible for disease susceptibility (9).
In this paper, we describe the generation of PLP-139–151-specific TCR-transgenic mice by expressing rearranged TCR α and β chains from an encephalitogenic and a nonencephalitogenic T cell clone derived from SJL mice and that are specific for PLP-139–151. The transgenic mice expressing either the encephalitogenic or the nonencephalitogenic TCR developed spontaneous EAE in specific pathogen-free/viral antibody-free conditions (SPF/VAF). More importantly, the two lines of transgenic mice expressing TCR from the nonencephalitogenic clone developed spontaneous EAE with such a high frequency that they could not be maintained on the SJL background.
Materials and Methods
DNA Constructs and Generation of Transgenic Mice.
The cDNA sequences of the TCR α and β chains from the 5B6 and 4E3 PLP-139–151-specific T cell clones have previously been identified (10). PCR primers with flanking restriction sites were designed to amplify the rearranged Vα4Jα8,Vβ6Dβ2Jβ2.6 and Vα11Jα12,Vβ16Dβ2Jβ2.5 segments from genomic DNA of the 5B6 and 4E3 T cell clone, respectively. The PCR products were subcloned into the appropriate restriction sites of genomic TCR expression cassettes (11). The transgenic constructs were electroporated together with plasmids containing mCD4 and the neomycin-resistance marker gene, respectively, into 58 α−β− T cell hybridomas (12). After selecting the transfectants in medium with G418 (1 mg/ml), resistant cells were screened by flow cytometry for the expression of CD3, CD4, or Vβ6. Positive clones were examined for antigen specificity as described below. To generate transgenic mice, linearized TCR plasmids were injected into the pronuclei of fertilized FVB/N (H-2q) oocytes. To introduce the H-2s haplotype into the transgenic background, the transgenic founders were backcrossed for five generations onto SJL/J mice. Transgenic mice were identified by Southern blot analysis of tail DNA by using VJα and VDJβ probes derived from the TCR plasmids as described below. Alternatively, mice were screened by FACS analysis of CD4-, CD8-, or Vβ6-stained peripheral blood cells as described below. SJL mice that were heterozygous for the RAG-2 deficiency were obtained by crossing homozygous RAG-2-deficient mice for five generations onto the SJL/J background. The TCR-transgenic mice were subsequently intercrossed with SJL/J-RAG 2+/− mice. TCR-transgenic and RAG-2+/− litters were finally intercrossed to obtain mice that were TCR transgenic and homozygous for the RAG-2 deficiency. Mice were housed at the Harvard Institutes of Medicine SPF/VAF facility and maintained in autoclaved cages with autoclaved bedding, food, and water.
DNA Isolation and Southern Analysis.
Isolation and analysis of DNA were carried out essentially as described by Sambrook et al. (13). At least 10 μg of genomic DNA was digested with BamHI and EcoRI, separated on agarose gels, and transferred to nylon membranes. Filters were hybridized overnight at 65°C in buffer containing 32P-labeled random primed 4E3 or 5B6 transgene-specific VJα and VDJβ probes. The filters were subsequently washed twice in 0.2× SSPE, 0.1% SDS at 60–65°C for 10 min each and exposed to Kodak X-Omat AR film.
Flow Cytometry.
Single-cell suspension of spleens and thymi were prepared, and RBC were lysed by hypotonic shock. Thymocytes, spleen cells, or lymph node cells (1 × 106 per sample) were incubated in 50 μl of wash buffer (PBS/0.1% NaN3/1% FCS) containing 0.25 μg of anti-mouse CD32/CD16 (PharMingen) for 3 min at 4°C. The cells were washed and subsequently stained with FITC- or phycoerythrin-conjugated antibodies at 4°C for 30–45 min. At least 30,000 cells per sample were analyzed on a FACS-sort model flow cytometer (Becton Dickinson).
Peptide Antigens.
The following peptide antigens were used: PLP-139–151 (HSLGKWLGHPDKF) (14), PLP-178–191 (NTWTTCQSIAFPSK), and Nase-101–120 (EALVRQGLAKVAYVYKPNNT). The purity of the peptides was >90% as determined by HPLC.
T Cell Proliferation.
Splenocytes (4 × 105 per well) were cultured in triplicates in RPMI 1640 medium (BioWhittaker) supplemented with 10% heat-inactivated FCS in the presence of indicated dilutions of synthetic PLP-139–151 or control peptides and incubated for 4 days at 37°C. Incorporated thymidine was determined after adding 1 μCi of [3H]thymidine to each well during the last 16 h of culture. TCR transfectants (1 × 105 per well) and irradiated (3,300 rad) SJL splenocytes (5 × 105/well) as antigen-presenting cells were cultured as above with various dilutions of PLP-139–151 or control peptides. The culture supernatants were harvested 24 h later and tested for the production of IL-2 by ELISA as described below.
ELISAs.
The concentration of cytokines was determined in culture supernatants of TCR transfectants and splenocytes 24 h and 40 h, respectively, after stimulation with peptide PLP-139–151 by quantitative capture ELISA according to the manufacturers' guidelines. Assays were developed with TMB Microwell Peroxidase Substrate (Kirkegaard & Perry Laboratories) and read at 450 nm.
Induction and Assessment of EAE.
For the development of spontaneous EAE, unimmunized mice were examined routinely for signs of EAE. EAE was induced in the transgenic mice by three different protocols: mice were immunized with PLP-139–151 together with pertussis toxin (PT), PLP-139–151 alone, or PT alone. In the first protocol, mice were injected s.c. in each flank with 25 μg of PLP-139–151 emulsified in complete Freund's adjuvant (CFA; Difco) supplemented with 400 μg of Mycobacterium tuberculosis (H37RA; Difco). Each mouse was also injected i.v. with 75 ng of PT (List Biological Laboratories, Campbell, CA) in 0.1 ml of PBS immediately and 48 h after the immunization. In the alternative protocols, mice were injected with PLP-139–151 or PT only as above. Mice were examined daily for signs of EAE, which were graded as follows: flaccid tail, 1; uneven gait and impaired righting reflex, 2; total hindlimb paralysis, 3; fore- and hindlimb paralysis, 4; and moribund, 5. At the peak of the disease or at the end of the experiment, brains and spinal cords were removed and fixed in 10% formalin (Fisher Scientific) and examined histopathologically for inflammation and demyelination as described (15).
Immunohistochemistry.
At the time of sacrifice, brains and spleens of transgenic and nontransgenic mice were removed and snap frozen in OCT compound (Sakura Finetek U.S.A., Torrance, CA). Cryostat sections were immunostained with rat anti-mouse CD4 (clone RM 4-5) and rat anti-Vβ6 TCR (clone RR4-7) (both from PharMingen) by avidin-biotin immunoperoxidase, as described (15).
Results
Generation of PLP-Specific TCR Transgenic Mice.
We isolated genomic rearranged TCR genes from the PLP-139–151-specific T cell clones 5B6 and 4E3 (16) to generate TCR transgenic mice. Both the 5B6 and 4E3 clones are CD4+, I-As-restricted, and specific for PLP-139–151, but there are important differences between them. Both clones reacted against the same truncated PLP peptides spanning PLP-139–151, but, in addition, the 5B6 T cell clone responded to the smaller truncated peptide PLP-139–145 (16). The proliferative response of the 5B6 but not the 4E3 T cell clone to PLP-139–151 could be blocked by anti-CD4 mAb, suggesting that the CD4 molecule on the surface of the 4E3 clone was nonfunctional (16). The 4E3 and the 5B6 T cell clone both produced Th1 cytokines following antigen stimulation, but the 5B6 clone showed a much higher expression of TCA3 chemokine mRNA compared with the 4E3 clone. Furthermore, the 5B6 T cell clone was cytolytic in vitro and strongly encephalitogenic in vivo, whereas the 4E3 clone was noncytolytic and could not transfer EAE (17).
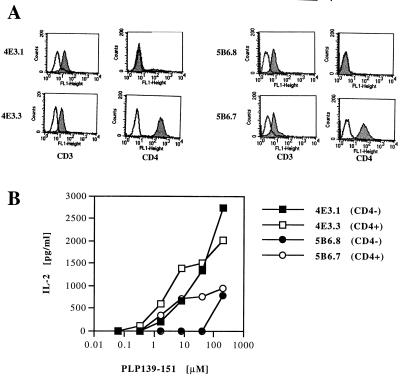
The rearranged TCR genes, which utilized Vα4 and Vβ6 (5B6 clone) and Vα11 and Vβ16 (4E3 clone) (10), were subcloned into cosmid cassette vectors containing TCR-specific regulatory elements (11). To examine the functionality and specificity of the cloned TCR plasmids, the transgenic constructs were electroporated with plasmids containing mCD4 and with the neomycin-resistance marker gene into the 58 α−β− T cell hybridoma (12). G418-resistant cells were screened for expression of CD3, CD4, and TCR by flow cytometry (Fig. 1A). Positive transfectants that expressed CD3/TCRs with and without CD4 showed antigen-induced IL-2 production in the presence of PLP-139–151 and SJL splenocytes as antigen-presenting cells (Fig. 1B). Previous studies have shown that the T cell response to a low affinity TCR/MHC ligand interaction depends more on coreceptor engagement than those to a high affinity interaction (18). To obtain an estimate of the relative affinity of the 5B6 and 4E3 TCRs for PLP-139–151, we examined the CD4 coreceptor dependence of transfectants expressing these TCR with or without CD4 coexpression in IL-2 production assays following antigen-specific activation (Fig. 1B). The CD4-negative, 5B6 TCR-expressing transfectants produced low amounts of IL-2 with a maximum (700 pg/ml) only at the highest antigen concentration tested. Coexpression of CD4 with the 5B6 TCR resulted in sustained production of low amounts of IL-2 from the transfectants at lower doses of antigen. The transfectants expressing TCR derived from the nonencephalitogenic 4E3 clone were able to recognize PLP-139–151 peptide and produce relatively large amounts of IL-2 (>2,000 pg/ml) even in the absence of CD4 expression. Coexpression of CD4 only marginally increased IL-2 production at lower doses of PLP-139–151 (Fig. 1B). These data show that both of the TCR constructs were functional and maintained their antigen specificity to PLP-139–151. Furthermore, the data indicate that the TCR derived from the nonencephalitogenic 4E3 clone was less dependent on CD4 coreceptor function, which may indicate that it has a relatively higher affinity for PLP-139–151/I-As than the TCR of the encephalitogenic T cell clone 5B6.
Figure 1.
Analysis of 4E3 and 5B6 TCR transfectants in 58 α− β− cell line. (A) Expression of CD3 and CD4 on 4E3 and 5B6 TCR transfectants. Expression of CD3 and CD4 was determined by flow cytometry by using FITC-conjugated anti-CD3 and anti-CD4 antibodies (filled histograms). Control stains using FITC-coupled isotype antibodies are shown (open histogram). (B) CD4 dependence of 4E3 and 5B6 TCR transfectants. CD4+ or CD4− 4E3 and 5B6 TCR transfectants were cultured with irradiated SJL splenocytes and stimulated with indicated concentrations of PLP-139–151. The concentration of IL-2 in supernatants of these cultures was determined by ELISA. Data from one of two experiments are shown.
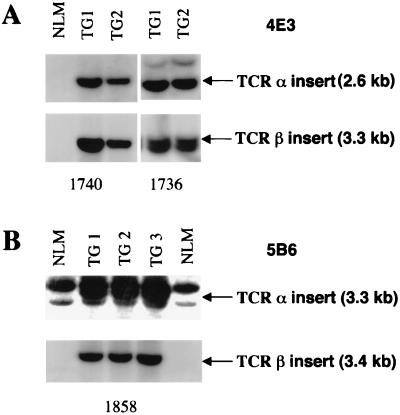
Transgenic mice were generated by coinjection of the linearized 5B6 and 4E3 TCR αβ constructs into pronuclei of FVB/N (H-2q) fertilized oocytes (19). A total of six TCR αβ DNA-positive founder mice were obtained, four expressing the 4E3 TCR constructs and two expressing the 5B6 TCR constructs. Two 4E3-specific lines and one 5B6-specific line showing germ-line transmission of the transgenes were selected for further analysis (Fig. 2). The TCR transgenes were introduced onto the H-2s background by successive backcrossing to SJL/J mice.
Figure 2.
Southern blot analysis of BamHI/EcoRI-digested tail DNA from 4E3 (A) and 5B6 (B) transgenic offspring. The TCR αβ transgenes of transgenic (TG) offspring from lines 1736, 1740 (4E3), and 1858 (5B6) are indicated by arrows. Nontransgenic littermates (NLM) are shown as controls. Southern blot analysis was carried out as described by using 32P-labeled 4E3 and 5B6 transgene-specific VJα and VDJβ probes.
Selection of T Cell Populations in PLP-TCR-Transgenic Mice.
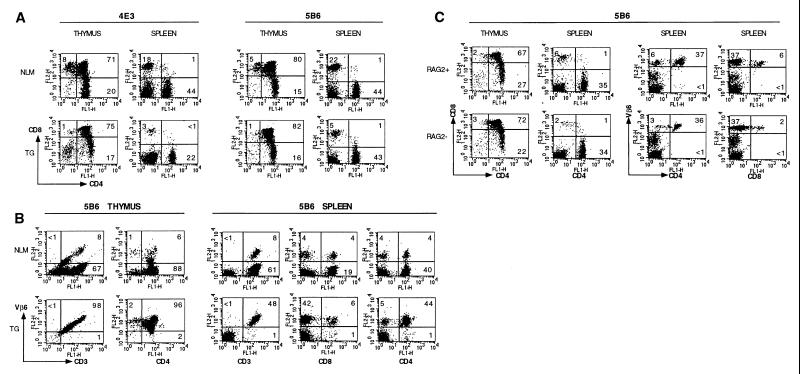
The thymi and spleens of transgenic animals were comparable in size to those of nontransgenic littermates. There was no significant difference in the number of T cells in the thymus or spleen of the transgenic mice compared with nontransgenic littermates, indicating that there was no gross clonal deletion of transgenic T cells (data not shown). As shown in Fig. 3, the CD4/CD8 ratio of thymocytes in PLP TCR-transgenic mice was increased compared with the nontransgenic littermates. The increase of the CD4/CD8 ratio is a pattern indicative of positive selection of the CD4+ transgenic T cells (20). Because the 5B6 TCR is composed of Vβ6, we analyzed the selection and expression of Vβ6-bearing cells in the thymus and peripheral lymphoid tissues of the transgenic mice by using the available anti-TCR Vβ6 antibody. For the 4E3 TCR, neither the clonotypic nor the specific Vα or Vβ antibody is currently available for this type of analysis. Essentially all (>98%) of the CD3+ thymic and splenic cells expressed the TCR Vβ6 chain in the 5B6 transgenic mice (Fig. 3B). These data demonstrate that, in the 5B6 TCR-transgenic mice, the vast majority of the mature T cells in the periphery expressed the transgenic TCR Vβ6. Establishing 5B6 TCR-transgenic mice that were RAG-2 deficient further substantiated this. The 5B6 TCR-transgenic T cells on the RAG-2−/− background were positively selected in the thymus and did not undergo significant deletion in the periphery when compared with the TCR transgenic RAG-2+/+ background and were highly responsive to PLP-139–151 in vitro (Fig. 3C, and data not shown).
Figure 3.
Flow cytometry analysis of thymocytes and peripheral T cells in TCR-transgenic mice. T cells from thymi and spleens of 6- to 8-week-old TCR transgenic 4E3 (line 1740, A), 5B6 (line 1858, A/B), and 5B6 RAG-2-deficient mice (line 1858, C) were stained with indicated antibodies (FITC- or PE-conjugated anti-CD4, FITC- or phycoerythrin-conjugated anti-CD8, PE-conjugated anti-Vβ6, FITC-conjugated anti-CD3). Dot plots representing two-color FACS analysis of TCR transgenic (TG) with or without RAG-2 deficiency (TG/RAG2−, TG/RAG2+) and nontransgenic littermates (NLM) are shown. Numbers in quadrants refer to percentages of gated cell populations.
T cells in the TCR transgenic mice specifically respond to PLP-139–151 peptide and produce Th1 cytokines.
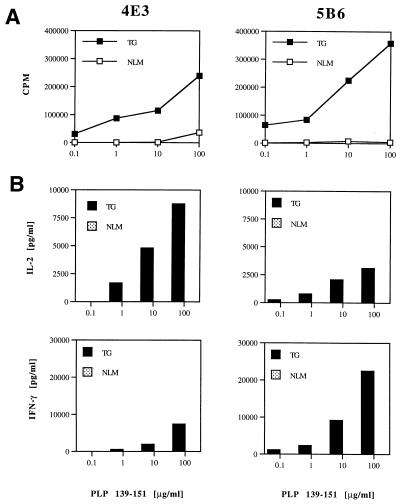
Splenocytes from unimmunized 5B6 and 4E3 TCR-transgenic mice proliferated vigorously when stimulated with PLP-139–151. This response was highly antigen specific because these splenic cells failed to proliferate to control I-As-binding peptides PLP-178–191 or Nase (data not shown). In contrast, PLP-139–151-specific proliferation was not detected in spleen cells obtained from naive nontransgenic littermates (Fig. 4A). Supernatants of proliferation cultures were assayed for secretion of IL-2, IL-4, IL-10, TNF-α, and IFN-γ by quantitative cytokine ELISA analysis. Consistently, we found that transgenic splenic T cell cultures stimulated with PLP-139–151 produced IL-2 and large amounts of IFN-γ compared with cells from nontransgenic littermates (Fig. 4B). The amounts of IL-4, IL-10, and TNF-α were generally below the detection limit in supernatants from both the transgenic and negative littermate cultures.
Figure 4.
Responses of transgenic T cells to PLP-139–151. (A) Proliferative response of T cells from 4E3- and 5B6-transgenic mice to PLP-139–151. Splenocytes from unimmunized 4E3 (line 1740) and 5B6 TCR-transgenic (line 1858) and nontransgenic littermate (NLM) mice were cultured with indicated concentrations of PLP-139–151 for 4 days. Proliferative responses were determined by [3H]thymidine incorporation analysis. (B) Transgenic T cells respond to PLP-139–151 by IL-2 and IFN-γ production. Cultures of splenocytes from 4E3 (line 1740) and 5B6 TCR-transgenic (line 1858) and nontransgenic littermate (NLM) mice were stimulated with PLP-139–151 for 40 h. Culture supernatants were assayed in duplicates for cytokine production by ELISA. Data from one of three experiments are shown.
Peripheral activation of transgenic T cells could not be analyzed directly because of the lack of anticlonotypic antibodies for the 4E3 and 5B6 TCRs. However, gating on CD4+ and CD4+Vβ6+ T cells from 4E3- and 5B6-unimmunized TCR-transgenic mice, respectively, revealed that these populations had an activated phenotype in some mice as assessed by staining for CD62L and CD69 or CD44 (data not shown).
Taken together, these data indicate that T cells from unimmunized transgenic mice are not tolerized and appear to be differentiated in vivo because they responded vigorously to the autoantigen PLP-139–151 by proliferation and production of IFN-γ upon stimulation.
PLP-TCR Transgenic Mice Develop a High Incidence of Spontaneous Autoimmunity of the CNS.
Because we backcrossed the transgenic mice onto the SJL background, it was possible to observe the transgenic mice on different backcross generations for the development of EAE for up to 1 year. During this period we detected spontaneous EAE during routine care and breeding of these mice. Under SPF/VAF conditions with autoclaved food and water, both 5B6 and 4E3 TCR-transgenic mice developed spontaneous EAE. Spontaneous disease in the transgenic mice was detected in each backcross generation and was specific for the transgenic mice, because we did not observe any signs of spontaneous disease in nontransgenic littermates. As shown in Table 1, 5B6 TCR-transgenic mice developed EAE spontaneously in all backcross generations (N2–N6) at an overall average of 40%. The frequency varied greatly between the N2 and N6 generations, but it was possible to breed the 5B6 TCR-transgenic mice even when 67% of the transgenic mice (in the N4 generation) showed signs of EAE. In contrast, two 4E3-transgenic mouse lines (1736 and 1740) developed increasingly a greater incidence of spontaneous EAE as they were backcrossed onto the SJL background, and both of these lines were lost before the N6 generation (Table 1). The disease was so prevalent that, by the N3 (line 1736) and the N5 (line 1740) generations, the majority (83% and 60%, respectively) of the mice developed EAE, and those mice that did not show clinical disease did not produce any offspring. Therefore, neither of the 4E3 lines could be maintained on the SJL background. This is in contrast to the frequency of spontaneous EAE in the 5B6-transgenic mice, which did not consistently increase with increasing numbers of backcrosses. Thus, we were able to maintain only the 5B6 TCR-transgenic mice on the SJL background. Because female SJL mice are more susceptible to induced EAE (21), we tried to ascertain whether there was a gender bias in the susceptibility to EAE in the TCR-transgenic mice. This gender bias was also seen in the spontaneous disease of the 1736-transgenic line where only female mice developed spontaneous EAE. However, the 1740 and 1858 lines did not show a female bias, because about equal numbers of males and females developed spontaneous disease (Table 1).
Table 1.
Frequency of spontaneous EAE in PLP TCR-transgenic mice
| Generation | 4E3
|
5B6
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1736
|
1740
|
1858
|
|||||||
| Incidence | Severity | Gender | Incidence | Severity | Gender | Incidence | Severity | Gender | |
| F1 | 1/13 (8%) | 2.5 | 1F | 0/7 (0%) | N/A | N/A | 0/4 (0%) | N/A | N/A |
| N2 | 1/6 (16%) | 2.0 | 1F | 3/11 (27%) | 1.7 | 3F | 3/12 (25%) | 1.5 | 1M, 2F |
| N3 | 5/6 (83%) | 2.0 | 5F | 1/7 (14%) | 3.0 | 1M | 2/5 (40%) | 2.8 | 2F |
| N4 | N/A | N/A | N/A | 1/8 (13%) | 3.0 | 1M | 6/9 (67%) | 2.4 | 2M, 4F |
| N5 | N/A | N/A | N/A | 9/15 (60%) | 2.2 | 7M, 2F | 8/28 (29%) | 2.2 | 4M, 4F |
| N6 | N/A | N/A | N/A | N/A | N/A | N/A | 9/20 (45%) | 1.9 | 4M, 5F |
4E3 (1736, 1740) and 5B6 (1858) TCR-transgenic mice on indicated SJL backcross generations were monitored regularly for signs of EAE for at least 6 months. Numbers of transgenic mice with spontaneous EAE per total number of transgenic mice in each generation are shown, and resulting frequencies of spontaneous EAE are given in percentages (%). Severity is shown as mean peak disease severity. M, male; F, female; N/A, not applicable.
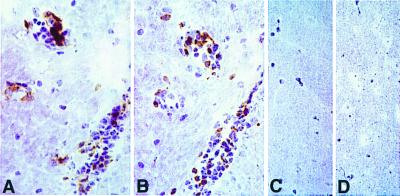
Pathological analysis of brain and spinal cord tissues from six representative transgenic mice with spontaneous disease showed typical histopathological evidence of EAE, i.e., numerous inflammatory foci in the meninges and CNS parenchyma. In contrast, analysis in transgenic mice with no clinical signs and nontransgenic littermates revealed no inflammation of the CNS (data not shown). To identify the CNS-infiltrating cells in transgenic mice with spontaneous EAE, we stained frozen sections of CNS tissue from 5B6-transgenic mice with anti-CD4 and anti-Vβ6 mAb antibodies. Similar numbers of cells in inflammatory lesions stained with each antibody, indicating that transgenic CD4+ Vβ6+ T cells were the predominant infiltrating cells in the CNS lesions (Fig. 5). In contrast, no CD4+ Vβ6+ cells were found in CNS sections from transgenic mice without signs of spontaneous disease (Fig. 5) or in nontransgenic littermate controls. These data demonstrate that CD4+,Vβ6+ cells readily infiltrate the CNS of the 5B6 TCR-transgenic mice with spontaneous EAE and that the spontaneous disease is not due to an expansion of nontransgenic (non-Vβ6)-bearing cells.
Figure 5.
Infiltration of transgenic Vβ6+ cells in the CNS of a transgenic mouse with spontaneous EAE. A and B are adjacent serial sections immunostained for CD4 (A) and Vβ6 (B) from a 5B6 TCR-transgenic mouse with spontaneous EAE. Similar numbers of perivascular inflammatory cells are stained in each. C and D are sections from the brain of a TCR-transgenic mouse, which did not develop spontaneous EAE, stained for CD4 and Vβ6, respectively. (All sections are stainings with immunoperoxidase with hematoxylin; (×514.)
Taken together, these results indicate that PLP-specific TCR-transgenic mice are prone to developing spontaneous EAE under SPF/VAF conditions as early as 6 weeks after birth and show the infiltration of transgenic T cells in their CNS.
PLP-Specific TCR-Transgenic Mice Are Very Sensitive to EAE Induction.
We examined the induction of EAE in 5B6-transgenic mice by using three different protocols. We first determined whether EAE could be induced by administration of PLP-139–151 in CFA. As shown in Table 2, the 5B6-transgenic mice developed more severe signs of EAE and at earlier time points than the nontransgenic littermate controls. Because the number of potentially autoreactive T cells in the transgenic mice is very large, we next determined whether EAE could be induced in these mice by the administration of PT alone. Injection of PT alone induced severe EAE in the majority of the transgenic mice within 11 days, whereas none of the nontransgenic littermates developed disease. Finally, we examined the development of EAE following immunization with PLP-139–151 in CFA and administration of PT. The mean onset of disease in both transgenic and nontransgenic mice was earlier than with the previous regimens. The transgenic mice showed the first signs of disease 1 week sooner than their nontransgenic littermates, and the average EAE severity was also greater than in the controls (Table 2). In summary, the transgenic mice were very sensitive to induction of EAE by administration of PT alone or by immunization with PLP-139–151 in CFA or the combination of PLP/CFA and PT.
Table 2.
PLP TCR-transgenic mice are very sensitive to induction of EAE
| Mice | Incidence of Clinical Disease | Mean Day of Onset | Mean Peak Disease Severity |
|---|---|---|---|
| PLP/CFA | |||
| TG | 7/7 | 11.9 ± 7.3∗ | 2.6 ± 0.9† |
| NLM | 4/5 | 27.0 ± 6.6 | 1.4 ± 0.5 |
| PT | |||
| TG | 5/6 | 10.6 ± 6.5 | 2.1 ± 0.7 |
| NLM | 0/6 | N/A | N/A |
| PLP/CFA/PT | |||
| TG | 7/7 | 7.2 ± 0.8‡ | 3.7 ± 1.0§ |
| NLM | 6/6 | 14.5 ± 3.2 | 2.3 ± 0.4 |
5B6 TCR transgenic (TG) and nontransgenic littermate (NLM) mice were injected with PLP-139–151 in CFA (PLP/CFA) s.c. or PT i.v. alone or in combination (PLP/CFA/PT). EAE was monitored for 40 days. Mean day of onset and mean peak disease severity are shown with SD. ∗, P = 0.008 versus NLM; †, P = 0.04 versus NLM; ‡, P = 0.0001 versus NLM; §, P = 0.008 versus NLM (all Student's t test, unpaired).
Discussion
In this paper, we describe the generation of TCR-transgenic mice with specificity for the major encephalitogenic epitope of the myelin protein PLP. The vast majority of mature T lymphocytes in the transgenic mice are CD4+ T cells that recognize PLP-139–151 in the context of I-As. Mature PLP-specific T cells are found in the periphery of these mice and are fully responsive to antigen. By crossing the 5B6-transgenic mice with RAG-2-deficient SJL mice, we could formally show that at least the 5B6 transgenic T cells are not deleted in the thymus or in the periphery. The lack of clonal deletion of myelin-reactive cells in the thymus was previously attributed to sequestration of myelin antigens in the nervous system and due to the lack of exposure of the myelin-reactive T cells in the thymus to the myelin auto-antigens. However, recent data suggest that both myelin MBP and PLP are expressed in the thymus (22) and peripheral lymphoid tissue (23, 24), raising the question of how MBP- and PLP-reactive T cells escape thymic deletion. Data from MBP-deficient shiverer mice suggest that expression of MBP in the thymus results in deletion of high affinity MBP-reactive T cells clones (25, 26). Our studies (27) and those of others (28) on thymic expression of PLP demonstrate that its smaller splice variant DM20, which lacks residues 116–150 and therefore essentially does not contain the PLP-139–151 epitope, is predominantly expressed in the embryonic thymus. Thus, restricted thymic expression of DM20, and not full length PLP, may explain why 5B6-transgenic T cells escape thymic deletion and seed in high numbers in the peripheral immune compartment.
The transgenic mice bearing TCRs from either encephalitogenic or nonencephalitogenic T cell clones developed spontaneous EAE. These results demonstrate that the specificity of the nonencephalitogenic TCR is sufficient to mediate EAE when expressed in vivo. It has been suggested that in EAE the TCR contributes to the encephalitogenicity not only by the recognition of myelin antigen in association of MHC, but also as an effector molecule (29). Our findings do not support this hypothesis because the transgenic mice bearing TCRs from either encephalitogenic or nonencephalitogenic T cell clones developed spontaneous EAE. These findings underscore the critical contribution of other factors responsible for CNS homing and initiation of tissue injury to the disease-mediating potential of autoreactive T cells. Although transgenic mice bearing TCRs from the encephalitogenic T cell clone developed EAE, the incidence of spontaneous EAE was much higher in mice bearing TCRs from the nonencephalitogenic clone. One possible explanation for this observation may be that the 4E3 TCR has a higher affinity for PLP-139–151/I-As complexes than the 5B6 TCR because the 4E3 TCR can recognize PLP-139–151/I-As even in the absence of CD4 molecules in vitro (Fig. 1). Thus the 4E3 TCR-bearing transgenic T cells may be activated in the peripheral immune compartment more readily, resulting in a higher frequency of activated T cells that induce a higher frequency of disease. Alternatively, one might argue that the frequency of transgenic T cells may be higher in the 4E3- than in the 5B6-transgenic mice because of the location of the transgene insertion, which could account for a higher incidence of spontaneous EAE in the 4E3-transgenic mice. Although we cannot directly exclude this possibility, it appears unlikely because we observed a high frequency of spontaneous disease in two different 4E3-transgenic lines. In a MBP TCR-transgenic system, infectious environmental agents were suggested as a cause for the observed development of spontaneous EAE, because the MBP-TCR-transgenic mice housed under conventional but not under SPF conditions developed EAE (30). In contrast, spontaneous disease in the PLP TCR-specific mice developed under VAF/SPF conditions in the absence of common viral and bacterial mouse pathogens. Whether the expansion/activation of PLP-139–151-reactive cells is due to the expression of PLP-139–151 in the periphery, or to a cross-reactive infectious agent or unrelated self antigen, requires further investigation. The data from the PLP TCR-transgenic mice described here differ from the findings in the previously reported MBP TCR-transgenic models in two major aspects. First, the frequency of spontaneous disease in the PLP TCR-transgenic lines is strikingly higher than the 14% reported in the MBP TCR-transgenic mice housed under SPF conditions (30). Second, immunization with antigen-adjuvant did not produce clinical signs in the MBP-transgenic mice but induced EAE readily in the PLP-transgenic mice under sterile housing. We do not know the reason for these differences between the two transgenic models, but they may reflect an intrinsic difference of the immune response to PLP versus MBP. The PLP-specific TCR-transgenic mice offer a valuable tool to gain new insights into mechanisms responsible for the dominance of the PLP autoantigen in the induction of EAE in SJL mice and the variation in disease susceptibility among various H-2s strains.
Acknowledgments
We thank Dianne Mathis and Bernard Malissen for the generous gifts of the TCR cassette vectors and the 58α−β− T cell hybridoma, respectively, and Arlene Sharpe for oocyte injections. We thank Heather Finnerty for DNA sequencing, Sheryl Henderson, Edward Howard, and Catherine Sabatos for technical help, and the members of the Collins and Kuchroo laboratories for stimulating discussions. We thank Leslie Berg, Arlene Sharpe, and Anthony Slavin for helpful comments on the manuscript. This work was supported by grants from the National Institutes of Health (ROI NS 30843, ROI NS 35685, and POI AI 39671) and the National Multiple Sclerosis Society (RG 2571 and RG 2320) to V.K.K., by a grant from the National Institutes of Health (NS 26773) to R.A.S., and by the Genetics Institute (M.J.W, M.C., and H.P.W.). H.P.W. has partly been supported by the Swiss Multiple Sclerosis Society and the National Multiple Sclerosis Society, New York.
Abbreviations
- PLP
proteolipid protein
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- MBP
myelin basic protein
- RAG
recombination-activating gene
- SPF
specific pathogen-free
- VAF
viral antibody-free
- PT
pertussis toxin
- CFA
complete Freund's adjuvant
References
- 1.Tuohy V K, Sobel R A, Lees M B. J Immunol. 1988;140:1868–1873. [PubMed] [Google Scholar]
- 2.Tuohy V K, Lu Z J, Sobel R A, Laursen R A, Lees M B. J Immunol. 1988;141:1126–1130. [PubMed] [Google Scholar]
- 3.Whitham R H, Jones R E, Hashim G A, Hoy C M, Wang R Y, Vandenbark A A, Offner H. J Immunol. 1991;147:3803–3808. [PubMed] [Google Scholar]
- 4.Amor S, Baker D, Groome N, Turk J L. J Immunol. 1993;150:5666–5672. [PubMed] [Google Scholar]
- 5.Greer J M, Sobel R A, Sette A, Southwood S, Lees M B, Kuchroo V K. J Immunol. 1996;156:371–379. [PubMed] [Google Scholar]
- 6.Greer J M, Kuchroo V K, Sobel R A, Lees M B. J Immunol. 1992;149:783–788. [PubMed] [Google Scholar]
- 7.Whitham R H, Bourdette D N, Hashim G A, Herndon R M, Ilg R C, Vandenbark A A, Offner H. J Immunol. 1991;146:101–107. [PubMed] [Google Scholar]
- 8.Kennedy M K, Tan L J, Dal Canto M C, Tuohy V K, Lu Z J, Trotter J L, Miller S D. J Immunol. 1990;144:909–915. [PubMed] [Google Scholar]
- 9.Encinas J A, Lees M B, Sobel R A, Symonowicz C, Greer J M, Shovlin C L, Weiner H L, Seidman C E, Seidman J G, Kuchroo V K. J Immunol. 1996;157:2186–2192. [PubMed] [Google Scholar]
- 10.Kuchroo V K, Collins M, al-Sabbagh A, Sobel R A, Whitters M J, Zamvil S S, Dorf M E, Hafler D A, Seidman J G, Weiner H L, et al. J Exp Med. 1994;179:1659–1664. doi: 10.1084/jem.179.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouskoff V, Signorelli K, Benoist C, Mathis D. J Immunol Methods. 1995;180:273–280. doi: 10.1016/0022-1759(95)00002-r. [DOI] [PubMed] [Google Scholar]
- 12.Letourneur F, Malissen B. Eur J Immunol. 1989;19:2269–2274. doi: 10.1002/eji.1830191214. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 14.Tuohy V K, Lu Z, Sobel R A, Laursen R A, Lees M B. J Immunol. 1989;142:1523–1527. [PubMed] [Google Scholar]
- 15.Sobel R A, Kuchroo V K. J Immunol. 1992;149:1444–1451. [PubMed] [Google Scholar]
- 16.Kuchroo V K, Sobel R A, Laning J C, Martin C A, Greenfield E, Dorf M E, Lees M B. J Immunol. 1992;148:3776–3782. [PubMed] [Google Scholar]
- 17.Kuchroo V K, Martin C A, Greer J M, Ju S T, Sobel R A, Dorf M E. J Immunol. 1993;151:4371–4382. [PubMed] [Google Scholar]
- 18.Hampl J, Chien Y H, Davis M M. Immunity. 1997;7:379–385. doi: 10.1016/s1074-7613(00)80359-3. [DOI] [PubMed] [Google Scholar]
- 19.Taketo M, Schroeder A C, Mobraaten L E, Gunning K B, Hanten G, Fox R R, Roderick T H, Stewart C L, Lilly F, Hansen C T, et al. Proc Natl Acad Sci USA. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg L J, Pullen A M, Fazekas de St. Groth B, Mathis D, Benoist C, Davis M M. Cell. 1989;58:1035–1046. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- 21.Voskuhl R R, Pitchekian-Halabi H, MacKenzie-Graham A, McFarland H F, Raine C S. Ann Neurol. 1996;39:724–733. doi: 10.1002/ana.410390608. [DOI] [PubMed] [Google Scholar]
- 22.Pribyl T M, Campagnoni C, Kampf K, Handley V W, Campagnoni A T. J Neurosci Res. 1996;45:812–819. doi: 10.1002/(SICI)1097-4547(19960915)45:6<812::AID-JNR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 23.Mathisen P M, Pease S, Garvey J, Hood L, Readhead C. Proc Natl Acad Sci USA. 1993;90:10125–10129. doi: 10.1073/pnas.90.21.10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voskuhl R R. Immunol Rev. 1998;164:81–92. doi: 10.1111/j.1600-065x.1998.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 25.Harrington C J, Paez A, Hunkapiller T, Mannikko V, Brabb T, Ahearn M, Beeson C, Goverman J. Immunity. 1998;8:571–580. doi: 10.1016/s1074-7613(00)80562-2. [DOI] [PubMed] [Google Scholar]
- 26.Targoni O S, Lehmann P V. J Exp Med. 1998;187:2055–2063. doi: 10.1084/jem.187.12.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson A C, Nicholson L B, Legge K L, Turchin V, Zaghouani H, Kuchroo V K. J Exp Med. 2000;191:1–11. doi: 10.1084/jem.191.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein L, Klugmann M, Nave K A, Kyewski B. Nat Med. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- 29.Heber-Katz E, Acha-Orbea H. Immunol Today. 1989;10:164–169. doi: 10.1016/0167-5699(89)90174-6. [DOI] [PubMed] [Google Scholar]
- 30.Goverman J, Woods A, Larson L, Weiner L P, Hood L, Zaller D M. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]