Abstract
Lipid storage diseases are debilitating inherited metabolic disorders that stem from the absence of specific lysosomal enzymes that degrade selected lipids. Most characteristically, these disorders affect the nervous and the reticulo-endothelial systems, with massive organomegaly resulting from the presence of engorged, lipid-laden macrophages. In this issue of the JCI, Yildiz et al. describe the role of the ER-resident enzyme β-glucosidase 2 (GBA2) in mice (see the related article beginning on page 2985). Surprisingly, GBA2 deficiency leaves bile acid and cholesterol metabolism intact, instead causing lipid accumulation in the ER of testicular Sertoli cells, round-headed sperm (globozoospermia), and impaired male fertility.
Glycosphingolipid metabolism in mammalian cells is maintained by a group of synthetic and degradative enzymes that often act in different cell types and subcellular compartments to achieve homeostasis. The term glycosphingolipidoses refers to a group of inherited glycosphingolipid storage disorders in humans (reviewed in ref. 1) caused by mutations in enzymes that degrade specific lipids in the acidic pH of lysosomes. Clinically, these disorders present with widespread systemic manifestations, with the nervous, reticulo-endothelial, and pulmonary systems severely affected. Since glycosphingolipids are important in cell signaling and development, glycosphingolipidoses are often onset in infancy or childhood and may be fatal if left untreated.
Glycolipids do their bit for posterity
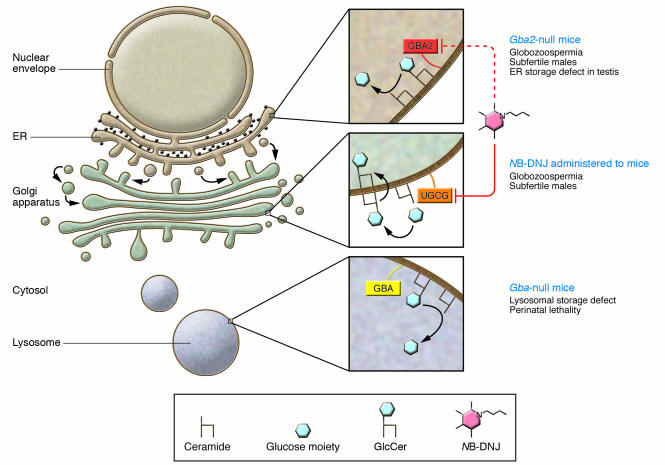
Several classes of glycolipids are essential for spermatogenesis, as demonstrated by spermatogenic failure in mice lacking either seminolipid or gangliosides (2, 3). Morphologically abnormal spermatozoa and impaired fertility are also seen in a mouse model of the lipid storage disorder Niemann-Pick disease, with lipid accumulation in testicular Sertoli cells (4). In humans, the most common glycosphingolipid storage disorder is Gaucher’s disease (OMIM 230800), which is caused by mutations in β-glucosidase acid (GBA; also known as glucosylceramidase) and results in glucosylceramide accumulation in lysosomes. Patients with Gaucher’s disease commonly exhibit hepatosplenomegaly and hematological abnormalities from glucosylceramide accumulation, but affected males can sire offspring. Unfortunately, Gba-null mice die perinatally (5), precluding studies on the effects of glucosylceramide accumulation on spermatogenesis (Figure 1). Current therapy for Gaucher’s disease involves either recombinant GBA enzyme replacement or administration of N-butyldeoxynojirimycin (NB-DNJ; also known as miglustat), an alkylated iminosugar that reduces glucosylceramide formation by inhibiting UDP-glucose ceramide glucosyltransferase, the resident Golgi apparatus enzyme that transfers glucose moieties to ceramide on the cytosolic face of the early Golgi compartment (6) (Figure 1).
Figure 1. Schematic showing subcellular localization and functions of enzymes involved in glucosylceramide metabolism.
Top inset: GBA2 hydrolyzes the glucose moiety from glucosylceramides (GlcCer) at the neutral pH of the ER lumen. A nonlysosomal glucosylceramidase (likely GBA2) is inhibited by NB-DNJ. Middle inset: UDP-glucose ceramide glucosyltransferase (UGCG) on the Golgi surface synthesizes glucosylceramide and is inhibited by NB-DNJ. After synthesis, glucosylceramide is flipped back into the Golgi lumen. Bottom inset: Glucosylceramides phagocytosed into the cell are normally degraded in the acidic pH of the lysosome by GBA. The phenotypes of Gba- and Gba2-null mice are described at right.
In this issue of the JCI, Yildiz et al. (7) show that the ER enzyme β-glucosidase 2 (GBA2) is also capable of acting as a glucosylceramidase in vivo in mice — a role hitherto exclusively ascribed to its more familiar lysosomal counterpart, GBA. The authors created a mouse line lacking Gba2 and report that bile acid and cholesterol metabolism as well as protein glycosylation in the testes of Gba2-null mice were minimally affected. However, a specific glycolipid — glucosylceramide — accumulated in Sertoli cells of Gba2-null testes. Incidentally, in patients with Gaucher’s disease, glucosylceramide is also the glycolipid that accumulates within and engorges hepatic and splenic macrophages (Figure 2). In contrast to patients with Gaucher’s disease, however, the male (but not the female) Gba2-null mice were subfertile, secondary to morphological defects of the sperm head (Figure 2), including round-headed sperm (globozoospermia).
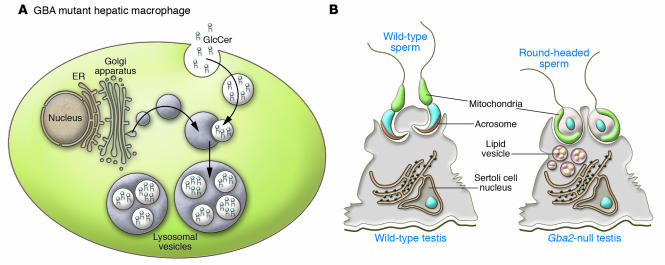
Figure 2. Different consequences of GBA and GBA2 deficiency.
(A) In patients with Gaucher’s disease, GBA deficiency leads to lysosomal accumulation of glucosylceramide and engorged hepatic and splenic macrophages. (B) In contrast, deficiency of GBA2 in mice leads to accumulation of lipid vesicles in testicular Sertoli cells. Dysmorphic round-headed sperm lacking acrosomes are produced in Gba2-null mouse testis compared with the normal falciform sperm heads in wild-type testis.
Intriguingly, NB-DNJ, a drug used to treat patients with Gaucher’s disease, has been recently shown to cause similar sperm head morphological defects and reversible male infertility in mice (8). Moreover, NB-DNJ is a potent inhibitor of an unidentified microsomal β-glucosidase activity in vitro (9). Since GBA2 is the only enzyme with a bona fide β-glucosidase activity known in the ER (7), this raises the possibility that the male contraceptive effect of NB-DNJ may be due at least in part to its inhibitory action on GBA2 in the testis.
Shaping the sperm head
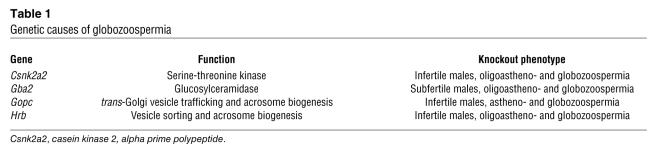
Globozoospermia in humans (OMIM 102530) and mice (Table 1) is a relatively rare defect of spermiogenesis in which round-headed sperm are produced with acrosomal defects and aberrant nuclear and flagellar morphology (10). Defective sperm acquire a round-headed morphology due to the nonextrusion of cytoplasmic droplets. Few genetic lesions causing globozoospermia are known, most of them (e.g., golgi-associated PDZ and coiled motif–containing protein [Gopc] and HIV-1 Rev–binding protein [Hrb]) affecting acrosome biogenesis in the germ cell (11, 12).
Table 1 .
Genetic causes of globozoospermia
In this context, Gba2 represents a Sertoli cell–expressed gene, the lack of which affects the modeling of the spermatid head. A central question is: How does an ER glucosidase in testicular Sertoli cells affect spermatid head shape? Yildiz et al. (7) suggest that disruption of germ cell–Sertoli cell glycolipid transport, with failure of glucosylceramide breakdown in the Sertoli cell ER of Gba2-null mice, may be a possible mechanism for the globozoospermia. Why or how the transported glycolipid would bypass the lysosomal degradative machinery en route to the ER is unclear. An alternative also proposed by the authors is the presence of a defect in lipid metabolism inherent to the Sertoli cell in which GBA2 fails to hydrolyze glucosylceramide in the ER lumen and consequently presents as an ER lipid storage disorder. It is worth noting that classical ER storage disorders are caused by errors in protein folding leading to targeted degradation and deficiency of those proteins. Also, for glucosylceramide to be processed by GBA2 in the Sertoli cell ER it must be retrogradely transported from an early Golgi complex, where it is normally synthesized, back to the ER; evidence for this event is currently lacking.
At present, direct effects of GBA2 deficiency on spermatids cannot be excluded since acrosome formation is defective in Gba2-null sperm (7). A similar defect in acrosome biogenesis accompanies the globozoospermia seen in mice null for either Hrb or Gopc (Table 1), and both genes are implicated in intracellular vesicular transport pathways that determine acrosome formation in spermatids. Also, lipid rafts (cholesterol-sphingolipid microdomains) on membrane bilayers are essential for normal intracellular vesicle trafficking (reviewed in ref. 13), and it is possible that GBA2 deficiency in spermatids impairs such vesicular transport by disrupting sphingolipid metabolism, ultimately causing globozoospermia. Experiments in which wild-type, GFP-positive spermatogonial stem cells are transplanted into Gba2-null testes, similar to a recent study (14), should provide unambiguous definition of the cellular compartments in which GBA2 plays an essential role.
Gba2 knockout and NB-DNJ: getting closer to a male contraceptive pill?
Several interesting points emerge from the current study by Yildiz et al. (7). First, the administration of NB-DNJ to mice partially phenocopies the reproductive deficits and the dysmorphic features in spermatozoa that are seen in Gba2-knockout mice. The fact that NB-DNJ treatment results in complete sterility, whereas Gba2-null mice are subfertile with a mean litter size of 2.8 (normal is approximately 8), suggests additive effects of other targets of this drug. This dichotomy can only be addressed by developing more specific antagonists of GBA2 or by identifying other targets of NB-DNJ, possibly by using Gba2-null mice. The second point addressed in this study relates to the putative role of Gba2 in mammals. Although GBA2 catalyzes bile acid glycosylation and hydrolysis in vitro and in vivo, cholesterol and bile acid metabolism is unperturbed in its absence, suggesting both functional redundancy and/or a lower phenotypic threshold preventing disease manifestation. Instead, what we believe to be a novel and essential glucosylceramidase activity of GBA2 was uncovered in the testis, underscoring the importance of dissecting gene function in vivo using knockout systems. In all probability, GBA2 represents the nonlysosomal glucosylceramidase activity that is intact in patients with Gaucher’s disease and was first identified in humans by Aerts and colleagues (15).
How does the contraceptive effect observed in Gba2 mutant mice translate to humans? NB-DNJ has been recently approved in Europe for treatment of a subset of patients with type I Gaucher’s disease who cannot tolerate or refuse GBA enzyme replacement (6). Prior to the discovery of its male contraceptive effect in mice, NB-DNJ was used in 2 human clinical trials, with very few adverse effects. In the first, it was used as an adjunct therapy with zidovudine for inhibiting viral replication in HIV-infected patients (16) at a dose of 3 g/d (equivalent to 43 mg/kg/d in a 70-kg person); in the other, it was used as substrate reduction therapy for Gaucher’s type I disease at 300 mg/d (equivalent to 4.3 mg/kg/d; ref. 17) as described above. The contraceptive effect of the drug observed in mice seems to occur at a dose of 5 mg/kg/d, therefore setting a benchmark for future clinical studies of the effects of this drug on contraception in humans. However, as the authors point out, Gba2 is more highly expressed in mouse testes than GBA2 is in human testes. Whether or not NB-DNJ has a similar contraceptive effect on humans therefore remains an open question.
In conclusion, results from the Gba2 knockout model (7) provide an interesting insight into glycolipid metabolism in the testis. In the future, this model may prove to be a valuable resource in the investigation of the male contraceptive effects of NB-DNJ, one of the prime candidates for the ever-elusive nonhormonal male contraceptive pill.
Acknowledgments
The authors thank Richard N. Sifers (Baylor College of Medicine) for his insightful comments and critical review of this commentary.
Footnotes
Nonstandard abbreviations used: GBA, β-glucosidase acid; GBA2, β-glucosidase 2; Gopc, golgi-associated PDZ and coiled motif–containing protein; Hrb, HIV-1 Rev–binding protein; NB-DNJ, N-butyldeoxynojirimycin.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:2860–2863 (2006). doi:10.1172/JCI30221.
Angshumoy Roy, Yi-Nan Lin, and Martin M. Matzuk contributed equally to this work.
See the related article beginning on page 2985.
References
- 1.Kolter T., Sandhoff K. Sphingolipid metabolism diseases. Biochim. Biophys. Acta. 2006 doi: 10.1016/j.bbamem.2006.05.027. In press. [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto H., et al. Requirement of seminolipid in spermatogenesis revealed by UDP-galactose: ceramide galactosyltransferase-deficient mice. J. Biol. Chem. 2000;275:22623–22626. doi: 10.1074/jbc.C000200200. [DOI] [PubMed] [Google Scholar]
- 3.Takamiya K., et al. Complex gangliosides are essential in spermatogenesis of mice: possible roles in the transport of testosterone. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12147–12152. doi: 10.1073/pnas.95.21.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler A., et al. Reproductive pathology and sperm physiology in acid sphingomyelinase-deficient mice. Am. J. Pathol. 2002;161:1061–1075. doi: 10.1016/S0002-9440(10)64267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tybulewicz V.L., et al. Animal model of Gaucher’s disease from targeted disruption of the mouse glucocerebrosidase gene. Nature. 1992;357:407–410. doi: 10.1038/357407a0. [DOI] [PubMed] [Google Scholar]
- 6.Cox T.M., et al. The role of the iminosugar N-butyldeoxynojirimycin (miglustat) in the management of type I (non-neuronopathic) Gaucher disease: a position statement. J. Inherit. Metab. Dis. 2003;26:513–526. doi: 10.1023/a:1025902113005. [DOI] [PubMed] [Google Scholar]
- 7.Yildiz Y., et al. Mutation of β-glucosidase 2 causes glycolipid storage disease and impaired male fertility. . J. Clin. Invest. 2006;116:2985–2994. doi: 10.1172/JCI29224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Spoel A.C., et al. Reversible infertility in male mice after oral administration of alkylated imino sugars: a nonhormonal approach to male contraception. . Proc. Natl. Acad. Sci. U. S. A. 2002;99:17173–17178. doi: 10.1073/pnas.262586099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matern H., Heinemann H., Legler G., Matern S. Purification and characterization of a microsomal bile acid beta-glucosidase from human liver. J. Biol. Chem. 1997;272:11261–11267. doi: 10.1074/jbc.272.17.11261. [DOI] [PubMed] [Google Scholar]
- 10.Matzuk M.M., Lamb D.J. Genetic dissection of mammalian fertility pathways. Nat. Med. 2002;8(Suppl.):s33–s40. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- 11.Kang-Decker N., Mantchev G.T., Juneja S.C., McNiven M.A., van Deursen J.M. Lack of acrosome formation in Hrb-deficient mice. Science. 2001;294:1531–1533. doi: 10.1126/science.1063665. [DOI] [PubMed] [Google Scholar]
- 12.Yao R., et al. Lack of acrosome formation in mice lacking a Golgi protein, GOPC. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11211–11216. doi: 10.1073/pnas.162027899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikonen E. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 2001;13:470–477. doi: 10.1016/s0955-0674(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., et al. Testis-specific sulfoglycolipid, seminolipid, is essential for germ cell function in spermatogenesis. Glycobiology. 2005;15:649–654. doi: 10.1093/glycob/cwi043. [DOI] [PubMed] [Google Scholar]
- 15.Van Weely S., Brandsma M., Strijland A., Tager J.M., Aerts J.M. Demonstration of the existence of a second, non-lysosomal glucocerebrosidase that is not deficient in Gaucher disease. . Biochim. Biophys. Acta. 1993;1181:55–62. doi: 10.1016/0925-4439(93)90090-n. [DOI] [PubMed] [Google Scholar]
- 16.Fischl M.A., et al. The safety and efficacy of combination N-butyl-deoxynojirimycin (SC-48334) and zidovudine in patients with HIV-1 infection and 200-500 CD4 cells/mm3. J. Acquir. Immune Defic. Syndr. 1994;7:139–147. [PubMed] [Google Scholar]
- 17.Cox T., et al. Novel oral treatment of Gaucher’s disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 2000;355:1481–1485. doi: 10.1016/S0140-6736(00)02161-9. [DOI] [PubMed] [Google Scholar]