Abstract
Life-history theory proposes that organisms must trade-off investment in current and future reproduction. Production of ornamental display is an important component of reproductive effort that has rarely been considered in tests of allocation trade-offs. Male eastern bluebirds (Sialia sialis) display brilliant ultraviolet-blue plumage that is correlated with mate acquisition and male competitive ability. To investigate trade-offs between current reproductive effort and the future expression of a sexually selected ornament, we manipulated the parental effort of males by changing their brood sizes. We found that parents provisioned experimentally enlarged broods more often than reduced broods. As predicted by life-history theory, the change in parental effort had a significant effect on the relative plumage ornamentation of males in the subsequent year: males with reduced broods significantly increased in plumage brightness. Moreover, this change in plumage coloration had a direct effect on the timing of breeding in the following season: males that displayed brighter plumage in the year following the manipulation mated with females that initiated egg laying earlier in the season. These data indicate that male bluebirds must trade-off conserving energy for production of future ornamentation versus expending energy for current reproduction.
Keywords: sexual selection, life-history evolution, trade-offs, structural plumage, parental effort
1. Introduction
Life-history theory argues that organisms are constrained by finite time and energy such that increased expenditure on one activity will necessarily lead to reduced allocation to other activities (Williams 1966; Levins 1968). A classic example involves current and future reproductive success. Because reproduction is energetically expensive, animals must trade current reproduction for future reproduction (Williams 1966; Partridge & Harvey 1985). If sexually selected traits are costly, then producing or maintaining such ornaments will require an allocation of resources that could also be invested in other life-history variables (Kokko 1998) or, conversely, an increase in reproductive effort should lead to a reduction in the elaboration of ornamentation and reduced mating success in subsequent years. Although it has been argued that sexual displays require resource allocation and therefore should be regarded as life-history traits (Höglund & Sheldon 1998), researchers have rarely attempted to quantify the cost of producing sexually selected ornaments.
Life-history trade-offs are difficult to study because, within a population, individuals have different energy budgets and thus may employ different reproductive strategies (Resnick 1985). A level of investment in offspring that is a burden to one individual may not be so to another individual. Because trade-offs occur at the individual level, experimental manipulations are required to separate trade-offs from natural covariances between ornament expression and reproductive effort (Sheldon 1996). Experiments that increased sexual displays revealed trade-offs between the current display and both mortality and future sexual displays (Moller 1989, 1994; Mappes et al. 1996), and experimental manipulations of parental effort demonstrated trade-offs between current parental investment and future sexual displays (Gustafsson et al. 1995; Griffith 2000).
In this study, we tested for trade-offs between reproductive effort and production of ornamental plumage coloration in the eastern bluebird (Sialia sialis). Male eastern bluebirds exhibit bright ultraviolet (UV)-blue structurally based plumage colour. Bluebirds are socially monogamous songbirds with biparental care (Pinkowski 1978) and relatively small brood sizes (mode=4). In recent studies, we demonstrated that the UV-blue coloration of male eastern bluebirds is related to male reproductive success and competitive ability (Siefferman & Hill 2003, 2005). Thus, structural plumage coloration is likely to be a sexual signal in eastern bluebirds. Here, we experimentally manipulated offspring brood size to determine whether trade-offs occur between current parental effort and the magnitude of the ornamental coloration produced for the next year.
2. Field Methods
We studied a colour-marked population of eastern bluebirds on an 8 km2 study site from March to August 2000–2002 in Lee County, AL. In 2000 and 2001, we enlarged and reduced the size of broods by two nestlings. We paired nests with four or five eggs and a common hatch date and cross-fostered nestlings on the second day post-hatch. We observed parental provisioning rates to offspring using video cameras for four continuous morning hours when chicks were 7 days of age. In the year following the manipulation, we captured the same males to quantify plumage coloration. We used the date that his mate laid her first egg of the season as a proxy of the pairing date.
(a) Colour quantification
At the time of capture, feather samples were collected from males for colour analysis. We measured plumage reflectance from eight rump feathers of each individual with a spectrometer (S2000, Ocean Optics, FL), following the methods of Siefferman & Hill (2003). We summarized reflectance data by calculating three standard descriptors of reflectance spectra. Brightness was calculated as the summed reflectance from 300 to 700 nm. UV chroma was calculated as the ratio of the total reflectance in the UV range to the total reflectance of the entire spectrum (∫300–400/∫300–700). Hue was calculated as the wavelength (nm) corresponding to maximal reflectance (λmax).
(b) Statistical analyses
We combined the data from 2000 and 2001 because analysis of variance determined no significant effects of year on provisioning rates or change in plumage colour (all p>0.10). Because the first egg dates differed significantly by year (F1,30=9.30, p=0.005), we standardized the data to a mean of zero and s.d. of one. We used SPSS (v. 11.5 Chicago, IL) to analyse the data and all statistical tests were two-tailed. For analysing plumage measures (brightness, chroma and hue), we adjusted our alpha level to p≤0.026 to account for multiple comparisons of correlated data with a proportional Bonferroni correction.
3. Results
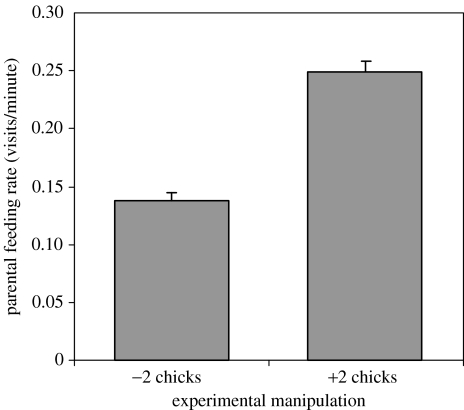
Analyses of provisioning videotapes indicated the brood size manipulation significantly changed (mean±s.e.) parental feeding rates; parents provisioned enlarged (X=5.6 chicks per nest) broods more than reduced (X=2.2 chicks per nest) broods (figure 1). There was no difference in the mean (s.e.) proportion of the provisioning rates provided by females and males (0.50±0.02 for females versus 0.51±0.02 for males; t=0.30, p=0.76, n=67, 67), and the proportion of the provisioning provided by each sex did not differ between the enlarged and reduced broods (females: 0.53±0.02 for enlarged versus 0.48±0.03 for reduced; t=1.2, p=0.22, n=37, 33).
Figure 1.
The mean feeding rate (number of feeding trips per minute) of eastern bluebird parents in relation to whether the brood size was reduced or enlarged (ANOVA: full model F3,64=33.6, p<0.001; experiment: F1,64=100.8, p<0.001). Bars represent s.e.
Next, we compared whether the proportional change in plumage colour from the year of the manipulation to the year following the manipulation (colour in year following manipulation/colour during manipulation year) differed between the birds that raised enlarged and reduced broods. There was a significant difference in the proportional change in brightness between the males in the two groups (1.15±0.07 versus 0.99±0.03; figure 2). Males that raised broods of reduced size increased in brightness while males that raised enlarged broods expressed colour that was the same or duller than in the prior year. However, there was no significant difference in (mean±s.e.) proportional changes in UV chroma (Student's t-test: 1.03±0.02 versus 1.00±0.02; t=0.95, p=0.33, n=11, 15), or hue (1.0±0.01 versus 0.99±0.01; t=0.39, p=0.70, n=11, 15) between the treatment groups. We also directly compared the paternal provisioning rates with the proportional change in plumage colour from the year of the manipulation to the year plus one. Males that provisioned more often became significantly duller in the year (Pearson correlation: brightness r=−0.50, p=0.02, n=21); however, there was no significant relationship between provisioning rates and proportional change in UV chroma or hue (r=−0.12, p=0.61, n=21; r=−0.13, p=0.59, n=21).
Figure 2.
The mean (±s.e.) proportional change in the brightness of the rump (brightness in year following manipulation/brightness during manipulation year) of male eastern bluebirds in relation to whether the brood size was either reduced or enlarged (Student's t-test: t=2.26, p=0.026, n=11, 15).
To determine whether the plumage coloration of males in the year following the manipulation influenced the pairing date in that breeding season, we ranked males by plumage coloration and compared the rank of each to the date that his mate laid her first egg of the season. Brighter males paired significantly earlier in the breeding season (rsr=−0.54, p=0.004, n=26); however, there was no significant relationship between first egg date and either UV chroma or hue (Spearman Rank Correlation: rsr=−0.15, p=0.48, n=26; rsr=0.05, p=0.81, n=26).
4. Discussion
Decreasing brood sizes caused male bluebirds to invest less in parental care and this reduction in reproductive effort had a significant positive effect on their expression of ornamental plumage coloration in the next breeding season. Moreover, male plumage coloration had a direct effect on the date that his mate initiated egg laying—females mated to brighter males initiated egg laying earlier than did the females paired to drabber males. The date of egg laying may indicate that brighter males paired earlier in the season. Alternatively, duller males may have paired with poorer quality females that take longer to acquire resources for egg-laying. Indeed, in this population, the females that lay eggs earlier in the season are either in better body condition or older (Siefferman, unpublished data). Overall, these observations support the idea that at least one component of male plumage coloration, in this case plumage brightness, is costly to produce and serves as an honest signal of male condition at the time of moult (Andersson 1994; Hamilton & Zuk 1982; Kodric-Brown & Brown 1984; Zahavi 1975). These observations further show that investment in secondary sexual traits is part of the overall energy budget of an organism and hence that sexually selected traits should be viewed in the same context as classic life-history traits in studies of resource allocation.
Bright UV-blue coloration in eastern bluebirds is likely to have evolved via sexual selection. Previous work with this population demonstrated that brighter and more UV-chromatic males paired earlier, provided more food to offspring and fledged offspring of larger size than did duller males (Siefferman & Hill 2003). Moreover, experiments have shown that more colourful males were better able to compete for access to nest cavities than were duller males (Siefferman & Hill 2005). Male aggression plays a central role in the breeding biology of this species because eastern bluebirds cannot excavate their own nesting cavities and thus males aggressively compete for access to nesting cavities. Signalling male competitive ability may be particularly important because males without a suitable nest-cavity cannot attract a mate.
Why should expression of the brightness but not the hue or UV chroma of the blue plumage of bluebirds be affected by reproductive effort? Eastern bluebird feathers are composed of a spongy medullary layer of feather barbs lying beneath a keratin cortex and above a layer of melanin granules surrounding large central vacuoles (Shawkey et al. 2003). The precision of structural elements within the spongy layer (such as the distance between scatterers and the distance between the spongy layer and the melanin granules from the cortex) determines the UV chroma and hue (Shawkey et al. 2003, in press). In contrast, brightness is predicted by the thickness of the keratin cortex that surrounds the medullary layer such that birds with brighter plumage have feathers barbs with a thinner cortex (Shawkey et al. in press). These observations suggest that males in better physiological condition during moult may be able to sequester the resources necessary to produce optimal cortex thickness. Thus, brightness appears to reliably signal condition while UV chroma and hue appear to be affected less by environmental influences.
UV-blue plumage brightness in male eastern bluebirds appears to be a costly trait that is maintained via sexual selection. Because of the likelihood of positive phenotypic covariances between the magnitude of display and measures of male fitness, it is very difficult to test the condition dependence of sexual traits without experimental manipulations. The evidence presented here indicates that models of sexual selection should take into account variation in the ability of individuals to bear the costs of developing and carrying ornaments, because these differences can apparently give rise to a positive association between the extent of development of the ornament and one or more components of fitness.
E. Gering and C. Ariail helped with data collection and R. Montgomerie allowed us to use his spectral processing program (ColouR v. 1.7). Comments from D. Broussard and the Hill lab group greatly improved this manuscript. This research was conducted according to an animal use permit from Auburn University and banding permits to G.E.H. The research was funded by NSF grants IBN 9722171, IBN 0235778, DEB 0077804, NIH/NSF programme in the Ecology of Infectious Diseases R01-AI49724 to G.E.H. and an Animal Behaviour Society grant to L.S. This experiment was conducted in accordance with the laws of the USA.
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection in animals. [Google Scholar]
- Griffith S.C. A trade-off between reproduction and a condition-dependent sexually selected ornament in the house sparrow Passer domesticus. Proc. R. Soc. B. 2000;267:1115–1119. doi: 10.1098/rspb.2000.1116. doi:10.1098/rspb.2000.1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson L, Qvarnström A, Sheldon B.C. Trade-off between life-history traits and a secondary sexual character in male collard flycatchers. Nature. 1995;375:311–313. [Google Scholar]
- Hamilton W, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- Höglund J, Sheldon B.C. The cost of reproduction and sexual selection. Oikos. 1998;83:478–483. [Google Scholar]
- Kodric-Brown A, Brown J. Truth in advertising: the kinds of traits favored by sexual selection. Am. Nat. 1984;124:309–323. [Google Scholar]
- Kokko H. Good genes, old age and life-history trade-offs. Evol. Ecol. 1998;12:739–750. [Google Scholar]
- Levins R. Princeton University Press; Princeton, NJ: 1968. Evolution in changing environments. [Google Scholar]
- Mappes J, Alatalo R, Kotiaho G, Parri S. Viability costs of condition-dependent sexual male display in a drumming wolf spider. Proc. R. Soc. B. 1996;263:785–789. [Google Scholar]
- Moller A.P. Viability costs of male tail length in a swallow. Nature. 1989;339:132–135. [Google Scholar]
- Moller A.P. Differential costs of secondary sexual character: an experimental test of the handicap principle. Evolution. 1994;48:1676–1683. doi: 10.1111/j.1558-5646.1994.tb02204.x. [DOI] [PubMed] [Google Scholar]
- Partridge L, Harvey P. The costs of reproduction. Nature. 1985;316:16–20. [Google Scholar]
- Pinkowski B. Feeding of nestling and fledgling eastern bluebirds. Wilson Bull. 1978;90:84–98. [Google Scholar]
- Resnick D. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. [Google Scholar]
- Shawkey M.D, Estes A.M, Siefferman L.M, Hill G.E. Nanostructure predicts intraspecific variation in ultraviolet-blue plumage colour. Proc. R. Soc. B. 2003;270:1455–1460. doi: 10.1098/rspb.2003.2390. doi:10.1098/rspb.2003.2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey M.D, Estes A.M, Siefferman L.M, Hill G.E. The anatomical basis for sexual dichromatism in non-iridescent ultraviolet-blue colouration of feathers. Biol. J. Linn. Soc. In press [Google Scholar]
- Sheldon B.C. Are bower bird displays cheap? Anim. Behav. 1996;52:645–647. [Google Scholar]
- Siefferman L, Hill G.E. Structural and melanin plumage coloration indicate parental effort and reproductive success in male eastern bluebirds. Behav. Ecol. 2003;14:855–861. [Google Scholar]
- Siefferman L, Hill G.E. UV-blue structural coloration and competition for nestboxes in male eastern bluebirds. Anim. Behav. 2005;69:67–72. doi:10.1016/j.anbehav.2003.12.026 [Google Scholar]
- Williams G.C. Natural selection, the costs of reproduction, and a refinement of Lack's principal. Am. Nat. 1966;100:687–690. [Google Scholar]
- Zahavi A. Mate selection—a selection for a handicap. J. Theor. Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]