Abstract
The desert locust Schistocerca gregaria is a well known migrating insect, travelling long distances in swarms containing millions of individuals. During November 2004, such a locust swarm reached the northern coast of the Gulf of Aqaba, coming from the Sinai desert towards the southeast. Upon reaching the coast, they avoided flying over the water, and instead flew north along the coast. Only after passing the tip of the gulf did they turn east again. Experiments with tethered locusts showed that they avoided flying over a light-reflecting mirror, and when given a choice of a non-polarizing reflecting surface and a surface that reflected linearly polarized light, they preferred to fly over the former. Our results suggest that locusts can detect the polarized reflections of bodies of water and avoid crossing them; at least when flying at low altitudes, they can therefore avoid flying over these dangerous areas.
Keywords: navigation, polarization vision, water avoidance, pest control, desert locust.
1. Background
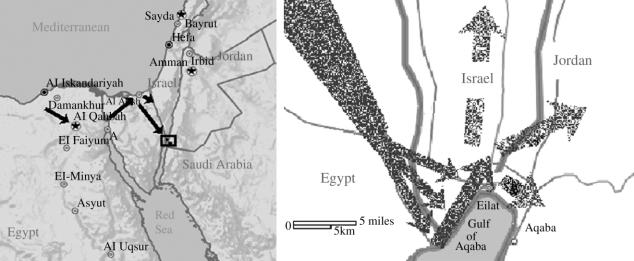
The desert locust Schistocerca gregaria is well known for its long-range migrations, with huge swarms travelling thousands of kilometres (Roffey & Magor 2003). A recent outbreak of locust migration included swarms crossing the length of the Sahara desert to reach Egypt, Jordan and Israel. During November 2004, a large locust swarm arrived, flying low from the Sinai desert towards the northern coast of the Gulf of Aqaba (the main swarm arrived between 19 and 23 November 2004, followed by smaller groups over the next 10 days). Upon reaching the coast, they did not cross the 3–5 km wide gulf, but instead turned north. Only after passing the northern tip of the gulf did the swarm turn again. A large section of the swarm turned east towards Jordan, while the rest continued north along the Arava valley (figure 1). Preliminary observations on the shore and from boats, performed by volunteers that tracked individual locusts in flight, confirmed that the insects rarely flew over water, and if blown over it rapidly turned back towards the shore. One should note that S. gregaria is well capable of crossing large bodies of water when flying high, often at a height of hundreds of metres, or when carried by strong winds (Symmons & Cressman 2001; Roffey & Magor 2003).
Figure 1.
Trajectories of locust swarms near the Gulf of Aqaba. Swarms of migrating desert locusts reached the northern Gulf of Aqaba from the Sinai peninsula on 21–23 November 2004, turned north when reaching the water, and crossed to Jordan, just over the northern coast of the gulf. Modified with permission from Haaretz news service.
How do locusts detect and recognize such water surfaces? Different forms of visual information are available to a flying insect that it may use to choose a particular direction in which to head. One of these is polarization vision. Light reflected from the sea is partly linearly polarized (Wehner 2001; electronic supplementary material) and hence polarization may provide a flying insect with an indication of the nature of the surface. Indeed, some insects use their polarization sensitivity to detect water bodies to which they are attracted (Schwind 1991; Horváth & Zeil 1996).
Locusts are known to be sensitive to linearly polarized light and to possess a specialized group of modified omatidia in the dorsal rim of their compound eyes (Eggers & Gewecke 1993; Mappes & Homberg 2003; Homberg et al. 2004), which are designed to analyse the pattern of polarized skylight as a navigational cue (Wehner 2001; Homberg 2004).
In the present work, we explore the possibility that polarization sensitivity can be used by locusts to navigate towards or away from surfaces such as large bodies of water, which reflect linearly polarized light (Schwind 1991; Horváth & Varjú 1997, 2003; Bernáth et al. 2002).
2. Methods
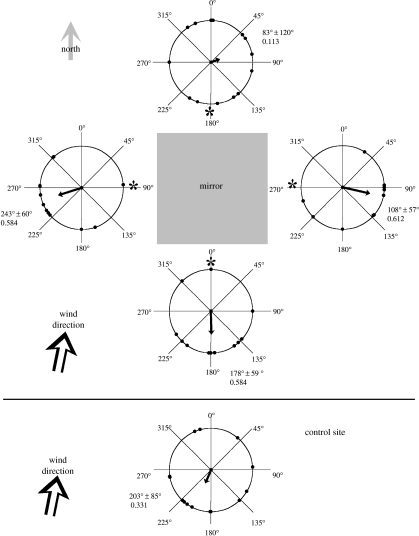
To test the response of locusts to reflecting surfaces, we examined their flight preference when they encountered a mirror. Fifteen individuals, freshly caught from the field, were tethered and released by hand close to and above a mirror on the ground, as well as at a nearby location without a mirror (figure 2). Locusts were released 10–15 cm above the ground, 5 cm away from the sides of a 1×1 m2 mirror (marked by asterisks in figure 2). The mirror was placed horizontally on the ground, under a completely blue sky in an open area with a light wind coming from the south (from compass bearing 190°). A naive observer (who was unaware of the purpose of the experiment) recorded the compass direction of flight of the tethered locusts after their release. The sides of the presentations were randomized. The locusts were tethered to 1 m long, thin, flexible strings, glued to the dorsal part of their thorax. The glue and string did not limit the animals' flying, jumping and crawling ability, and on some occasions they flew away with the strings attached. After the experiments, when the glue and string were removed, the released animals flew normally. The mirror reflected partially linearly polarized light at least across the 330–800 nm range (examined with a spectral polarimeter; Shashar et al. 2004).
Figure 2.
Locusts' flight direction around a 1×1 m2 mirror and in a control area. Arrows indicate mean vector of flight directions; numbers show mean vector directions±circular standard deviations and the length of the mean vectors; n=15 animals. The asterisk indicates the approximate place of the animals' release. Although locusts generally flew towards the wind, they avoided flying in the direction of the light-reflecting mirror or flew away from it.
Strong reflections from a mirror may cause flying insects to execute roll turns with subsequent changes in flight direction, triggered by their dorsal light response (Goodman 1965, Schuppe & Hengstenberg 1993). To determine whether the migrating locusts have a preference for flying towards a polarizing surface, we tested 40 tethered new locusts in the following experiment. Two 1.2×0.8 m areas of flat black material were placed on the ground with an exposed 10 cm wide gap between them. The gap was aligned away from the sun to ensure equal illumination on both squares. The test materials consisted of a linearly polarized light-reflecting plastic (brightness 25.2±11, 0–255 scale where 0 is black and 255 white; percentage of polarization=77±6%; average±standard, n=98 536 pixels) and felt that reflected less polarized light (brightness 21.5±10; percentage of polarization=46±3%, n=89 464 pixels), both examined with an imaging polarimeter (Shashar et al. 2004) from the position of the tested animal. Each black background was tested in equal numbers on each side of the gap. The setup was protected from natural wind and a commercial fan generated a light wind along the central gap, towards the sun, with a similar wind speed on both sides of the gap. The locusts were tethered to a preset apparatus positioned 50 cm above the centre of the gap and were manually released directly underneath it, 5–7 cm above the ground. Immediately after the insect's release, the experimenter moved away along the direction of the gap to a distant position. A digital video camera, placed at the height of the animals and facing towards the centre of the gap (the release point) recorded the flight paths. Frame-by-frame analysis was later used to determine the flight directions of the insects after they were released. The camera was positioned downwind in such a way that it did not generate shade on the experimental surfaces. Animals were tested individually and only once. Statistics were analysed using Statistica v.6.0 and Oriana v.2.0 software.
3. Results
An examination of the flight response in the vicinity of the mirror revealed that the locusts avoided flying towards the mirror from all release points, except the northern one (p<0.05; see figure 2 for the mean heading and circular standard deviation). At the northern release point, the wind came from the direction of the mirror and the lack of preferred orientation (Rayleigh test; p=0.841) may indicate a conflict between flying into the wind and avoiding the mirror (in nature locusts often fly towards the wind, if it is not too strong). The average flight direction in the control position without a mirror tended to coincide with the direction from which the wind was coming (203±85° versus 190°, respectively; figure 2). Examining the flight preference when presented with two similar black surfaces that differ in their polarization reflection revealed that the locusts did not have any side preference (18/22 right/left choice, χ2=0.5, p<0.53), yet flew significantly more often towards the less polarizing background than towards the polarizing one (28/12 respectively, χ2=6.4, p<0.015).
4. Discussion
Our results show that migrating locusts avoid flying over surfaces that have a strong linear polarization reflection. A response similar in nature but opposite in direction (i.e. attraction towards polarizing surfaces) was found in insects that live on or near bodies of water (Schwind 1991, 1995; reviewed in Horváth & Varjú 2003). For a migrating desert insect, water presents a potential hazard and large bodies of water, such as the sea, are especially dangerous. Therefore, detecting and avoiding such surfaces is beneficial to them.
Polarization sensitivity has so far been examined in connection with the dorsal rim area of the locust eye, which is involved in detecting a compass direction using the sky's polarization pattern (for review see Homberg 2004). Our results show that these insects are also able to detect polarized light in the ventral visual field. The underlying mechanism that enables this sensitivity is yet to be identified; it may involve anatomical or behavioural adaptations. The polarization signal itself may be perceived via either a response to light intensity at a given polarization orientation or via true polarization sensitivity (Cronin & Shashar 2001; Wehner 2001).
Polarized reflections may indeed help migrating locusts avoid flying over water in coastal areas. It is likely that this capacity will be most relevant to locusts when they are flying at low altitudes and when other weather conditions, such as wind speed and direction, permit flying in specific directions. It is also possible, though yet to be studied, that other polarization-reflecting materials could deter locusts as well.
Acknowledgments
We thank Barak Guzner for his assistance during the experiments, Uwe Homberg and Jeffery Camhi for their useful advice, Thomas W. Cronin, and two anonymous reviewers for improving this manuscript.
Supplementary Material
References
- Bernáth B, Szedenics G, Wildermuth H, Horváth G. How can dragonflies discern bright and dark waters from a distance? The degree of polarization of reflected light as a possible cue for dragonfly habitat selection. Freshwater Biol. 2002;47:1707–1719. [Google Scholar]
- Cronin T.W, Shashar N. The linearly polarized light field in clear tropical marine waters: spatial and temporal variation of light intensity, degree of polarization, and e-vector angle. J. Exp. Biol. 2001;204:2461–2467. doi: 10.1242/jeb.204.14.2461. [DOI] [PubMed] [Google Scholar]
- Eggers A, Gewecke M. The dorsal rim area of the compound eye and polarization vision in the desert locust (Schistocerca gregaria) In: Wiese K, Gribakin F.G, Popov A.V, Renninger G, editors. Sensory systems of arthropods. Birkhäuser Verlag; Basel: 1993. pp. 101–109. [Google Scholar]
- Goodman L.J. The role of certain optomotor reactions in regulating stability in the rolling plane during flight in desert locust Schistocerca gregaria. J. Exp. Biol. 1965;42:385–407. [Google Scholar]
- Homberg U. In search of the sky compass in the insect brain. Naturwissenschaften. 2004;91:199–208. doi: 10.1007/s00114-004-0525-9. [DOI] [PubMed] [Google Scholar]
- Homberg U, Hofer S, Mappes M, Vitzthum H, Pfeiffer K, Gebhardt S, Müller M, Paech A. Neurobiology of polarization vision in the locust Schistocerca gregaria. Acta Biol. Hung. 2004;55:81–89. doi: 10.1556/ABiol.55.2004.1-4.10. [DOI] [PubMed] [Google Scholar]
- Horváth G, Varjú D. Polarization pattern of fresh water habitats recorded by video polarimetry in red, green and blue spectral ranges and its relevance for water detection by aquatic insects. J. Exp. Biol. 1997;200:1155–1163. doi: 10.1242/jeb.200.7.1155. [DOI] [PubMed] [Google Scholar]
- Horváth G, Varjú D. Springer; Heidelberg: 2003. Polarized light in animal vision—polarization patterns in nature. [Google Scholar]
- Horváth G, Zeil J. Kuwait oil lakes as insect traps. Nature. 1996;379:303–304. [Google Scholar]
- Mappes M, Homberg U. Behavioral analysis of polarization vision in tethered flying locusts. J. Comp. Physiol. A. 2003;190:61–68. doi: 10.1007/s00359-003-0473-4. [DOI] [PubMed] [Google Scholar]
- Roffey J, Magor J.I. Desert locust technical series. Food and Agriculture organization (FAO) of the United Nations; Rome: 2003. Desert locust population dynamics parameters. 29 pp. [Google Scholar]
- Schuppe H, Hengstenberg R. Optical properties of the ocelli of Calliphora erythrocephala and their role in the dorsal light response. J. Comp. Physiol. A. 1993;173:143–149. [Google Scholar]
- Schwind R. Polarization vision in water insects and insects living on moist substrate. J. Comp. Physiol. A. 1991;169:531–540. [Google Scholar]
- Schwind R. Spectral regions in which aquatic insects see reflected polarized light. J. Comp. Physiol. A. 1995;177:439–448. [Google Scholar]
- Shashar N, Sabbah S, Cornin T.W. Transmission of linearly polarized light in sea water—implications for polarization signaling. J. Exp. Biol. 2004;207:3619–3628. doi: 10.1242/jeb.01187. [DOI] [PubMed] [Google Scholar]
- Symmons P.M, Cressman K. Food and Agriculture organization (FAO) of the United Nations; Rome: 2001. Desert locust guidelines—biology and behaviour. [Google Scholar]
- Wehner R. Polarization vision—a uniform sensory capacity? J. Exp. Biol. 2001;204:2589–2596. doi: 10.1242/jeb.204.14.2589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.