Abstract
Influences of climate on life history traits in natural populations are well documented. However, the implications of between-individual variation in phenotypic plasticity underlying observed trait–environment relationships are rarely considered due to the large, long-term datasets required for such analysis. Studies typically present correlations of annual trait means with climate or assume that individual phenotypic responses are constant. Here, we examine this additional level of variation and show that, in a red deer population on the Isle of Rum, Scotland, changes in climate generate changes in phenotype only amongst individuals who have experienced favourable ecological conditions. Examination of relationships between offspring birth weight and spring temperature within the lifetimes of individual females revealed that the tendency to respond to climate declined as the population density experienced early in life increased. The presence of such systematic variation in individual plasticity is rarely documented in the wild, and has important implications for our understanding of the environmental dependencies of traits under varying ecological conditions.
Keywords: phenotypic plasticity, constraint, life history, Cervus elaphus, climate
1. Introduction
The influence of climatic variation on life history traits in natural populations is well documented (Stenseth et al. 2002). Many observed relationships between life history traits and the environment can be attributed to individual organisms expressing different phenotypes across their lifetimes in response to the conditions they experience (or phenotypic plasticity; e.g. Both et al. 2004). Such life history plasticity at the individual level may be the result of condition-dependence: climate influences an individual's physiological condition, constraining the expression of costly traits or altering life history decisions (Stevenson & Bryant 2000). Changing environmental circumstances, such as increased resource competition, may also impact on individual physiological condition and thus alter individual responses to climate. However, the effect of environmental deterioration on individual responses to the environment in wild vertebrate populations remains unexplored.
To date, studies of vertebrate life history responses to the environment have typically assessed the correlation between annual population means for a given trait with an environmental variable (Post & Stenseth 1999; Both et al. 2004) or utilised models that assume a constant response of all individuals in the population (Przybylo et al. 2000). The presence of and reasons for individual variation in life history responses to climate are rarely examined; however, this variation will underpin any population's ability to track environmental change over a prolonged period (Nussey et al. 2005).
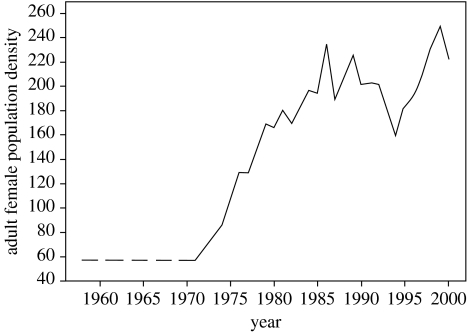
Here, we explore individual variation in a maternal life history trait (offspring birth weight)–climate (spring temperature) relationship in a wild population of red deer (Cervus elaphus) on the Isle of Rum, Scotland (Clutton-Brock et al. 1982). Previous studies of this population, treating birth weight as a trait of the offspring, have revealed that annual average birth weights are heavier following warm springs (Albon et al. 1987). This relationship is presumably driven by condition-dependence in maternal investment late in gestation: warm temperatures improve grazing conditions and hence pregnant females' physiological condition, allowing greater investment in foetal growth (Albon et al. 1987). A female's physiological condition may also be affected by the environmental conditions she experiences, and early experiences of environmental quality are known to have persistent effects on performance later in life (Kruuk et al. 1999; Post & Stenseth 1999). One of the key determinants of the quality of the environment in the Rum study area is adult female population density. Density has steadily increased through the 1970s following the cessation of culling in 1973, reaching carrying capacity in the early 1980s around which it has since fluctuated (figure 1). This increase in resource competition has generated declines in numerous measures of performance, such as juvenile survival and adult fecundity (Clutton-Brock et al. 1987; Kruuk et al. 1999). We examined the effects of this increase in population density and corresponding decline in environmental quality on the strength of the birth weight–spring temperature relationship within females.
Figure 1.
Line plot showing the female population density (number of resident females observed in more than 10% of January to May censuses of the study area over 1 year of age), an indicator of environmental quality in the study area, over time. Females born before regular censusing began were assumed to have experienced densities equal to those in 1971 (dashed line).
2. Methods
All data were collected on red deer in the North Block of the Isle of Rum, Scotland (a 12 km2 study area located 57°01′ N, 06°17′ W) between 1971 and 2000. Female deer give birth to a maximum of one offspring per year, usually in late May or June. Extensive daily surveys of the study population during this period meant that the timing of births was well known and most calves were caught and weighed within a few days of birth. Birth weights were calculated as follows: birth weight=capture weight (kg)−0.01539×age at capture (hours) (see Clutton-Brock et al. 1982 for further details). Weather variables were obtained from a Meteorological Office weather station on Rum, and spring temperature was defined as the average daily maximum temperature through the months of April and May (as in Albon et al. 1987). There was no evidence of a linear change in spring temperatures across the study period (F1,28=0.63, p=0.44).
Variation in offspring birth weights attributable to the significant effects of female's reproductive history (as a five level factor, defined following Coulson et al. 2003) and age (as a quadratic), as well as offspring sex and date of birth, was removed, and residual birth weights used in the analyses that follow. An individual female's plasticity was defined as the slope of a linear regression of her offspring's residual birth weight measurements on the spring temperatures she experienced in the year of each birth. Only females with measurements on four or more offspring were included in the analysis (190 out of 414 females). Mean plasticity and its coefficient of variation (as a percentage) were calculated.
We examined the effects of early experiences of population density on the strength of the birth weight–spring temperature relationship by grouping females according to the density in their year of birth (see figure 2), and regressing plasticity estimates on density. Population density was defined as the number of females of over 1 year of age that were present in more than 10% of January to May North Block censuses that year. Density estimates are available from 1971; however, many reproductive females in the data set were born earlier than this. Since the main cause of change in density over the study period has been the demographic consequences of a release from culling in 1973 (Clutton-Brock et al. 1982), we assume that density has been constant prior to 1971 (figure 1). In addition, we ran a mixed-effects model in S-Plus v. 6 (Insightful Corp.) on all available birth weight data (1557 observations from 414 different females). We included the terms used in the calculation of residual birth weights described above as fixed effects, and female identity as a random effect. The inclusion of the random effect for female means the model assesses the significance of fixed effects against variation in birth weight within females. We tested for changes in the dependency of birth weight on spring temperature with density by fitting spring temperature, density in a female's year of birth and their interaction to the model as fixed effects and assessing their direction and significance (see electronic supplementary material).
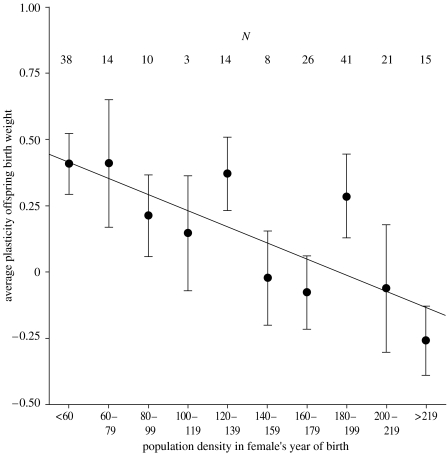
Figure 2.
The tendency for individual female red deer to give birth to heavier offspring following warm springs declines with deteriorating environmental conditions. The plot shows mean regression slope estimates (±s.e.) of individual females' residual offspring birth weight–spring temperature relationship (in kg °C−1) grouped by the densities that females experienced in their year of birth, with sample sizes for each density grouping indicated above the error bars. The regression line through these means is plotted (b=−0.0030 kg °C−1 female−1 ±0.0009 s.e.; p<0.01; r2=0.61).
3. Results
Estimates of individual plasticity confirmed that on average females gave birth to heavier offspring following warm springs: the average female offspring birth weight–spring temperature slope was 0.17 kg °C−1. There was considerable variation between females in the strength of their plastic response to spring temperature (coefficient of variation (%)=469.07).
There was a significant negative effect of density in year of birth on a female's plasticity (F1,8=12.55, p<0.01). Individual responses to spring temperatures declined as females experienced higher population densities early in life (figure 2). Plasticity decreased by 36% of its mean value for each additional 20 individuals present in the population in a female's year of birth. The generality of this reduction in plasticity with population density is substantiated by the presence of a highly significant negative interaction between spring temperature and population density in the mixed-effects model of birth weight including all available data (F1,1133=21.52, p<0.001; see electronic supplementary material).
4. Discussion
We have shown here that plastic responses to climatic variation may vary between individuals experiencing different ecological conditions. As the density of female red deer in the Rum study area increased to carrying capacity (figure 1), the relationship between offspring birth weight and spring temperature within individual females declined to zero (figure 2). There are several non-exclusive mechanistic explanations for the changes in birth weight–spring temperature correlations amongst individuals. Changes in individual plastic responses could explain the observed trend: reductions in birth weight plasticity with increasing density could be the result of adaptive changes in female investment, which reduce the risks to mothers of sustaining large foetuses when density is high and food is scarce. Also, reductions in plasticity may occur because high density reduces the variability of condition in females, limiting the ability of superior females to increase pre-natal investment in warm springs. An alternative explanation could be that individual responses to spring temperature are reduced because, as a result of increased grazing pressures, warm springs do not generate significant increases in primary productivity when density is high.
A variety of alternative explanations exist, but whatever the underlying mechanisms, the presence of systematic variation in individuals' plastic responses to climate has implications for the way we interpret trait–environment relationships in long-lived organisms. Correlation of annual mean offspring birth weights with spring temperatures in our study population would suggest that, should springs get warmer, offspring birth weights would increase. Analysis conducted here at the individual level implies that, as long as the population density remains high, systematic changes in spring temperatures will not affect offspring birth weights. Many studies examining annual trait means have likewise shown density-independent effects in wild vertebrate populations (see, for example, Post & Stenseth 1999; Both et al. 2004). Findings presented here at the individual level suggest that our ability to detect such environmental dependencies in life history traits may depend on the ecological conditions experienced by the population in question.
Analysis of variation in individual phenotypic plasticity in wild populations requires detailed information on individuals over long time series, and few studies will be able to meet these requirements. However, our analyses illustrate that the presence or absence of trait–climate relationships at the population level under one set of ecological conditions (such as rising population density) may not hold true under different conditions (such as a population at carrying capacity). Recent studies have documented variation in trait–climate correlations between geographically isolated populations (e.g. Both et al. 2004). Our findings hightight that whilst an effect of local ecological conditions on individual plasticity may explain observed variation between populations, it would also make extrapolation of results from one population to another problematic. Furthermore, in circumstances where there are adaptive benefits to environmental dependency in a given trait, a reduction in overall plasticity may be ultimately detrimental to population viability. By implication, populations may have to rely on the slower process of microevolution through a shift in their genetic composition to provide adaptation to changing environmental conditions.
Acknowledgments
The authors thank Scottish Natural Heritage for permission to work on Rum and their local staff for support. We thank Alison Donald, Sean Morris, and Fiona Guinness and many other field workers on the Kilmory deer project, as well as Tim Coulson, Alastair Wilson, and three anonymous referees for comments on earlier drafts of the manuscript. This work was supported by NERC (via a Ph.D. studentship to D.H.N., and research grants to T.H.C.B. and J.P.) and the Royal Society (L.E.B.K.).
Supplementary Material
References
- Albon S.D, Clutton-Brock T.H, Guinness F.E. Early development and population dynamics in red deer. II. Density-independent effects and cohort variation. J. Anim. Ecol. 1987;56:69–81. [Google Scholar]
- Both C, et al. Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc. R. Soc. B. 2004;271:1657–1662. doi: 10.1098/rspb.2004.2770. doi:10.1098/rspb.2004.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Guinness F.E, Albon S.D. University of Chicago Press; 1982. Red deer: the behaviour and ecology of two sexes. [Google Scholar]
- Clutton-Brock T.H, Major M, Albon S.D, Guinness F.E. Early development and population dynamics in red deer. I. Density-dependent effects on juvenile survival. J. Anim. Ecol. 1987;56:53–67. [Google Scholar]
- Coulson T.N, Kruuk L.E.B, Tavecchia G, Pemberton J.M, Clutton-Brock T.H. Estimating selection on neonatal traits in red deer using elasticity path analysis. Evolution. 2003;57:2879–2892. doi: 10.1111/j.0014-3820.2003.tb01528.x. [DOI] [PubMed] [Google Scholar]
- Kruuk L.E.B, Clutton-Brock T.H, Rose K.E, Guinness F.E. Early determinants of lifetime reproductive success differ between the sexes in red deer. Proc. R. Soc. B. 1999;266:1655–1661. doi: 10.1098/rspb.1999.0828. doi:10.1098/rspb.1999.0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussey D.H, Clutton-Brock T.H, Elston D.A, Albon S.D, Kruuk L.E.B. Phenotypic plasticity in a maternal trait in red deer. J. Anim. Ecol. 2005;74:387–396. doi:10.1111/j.1365-2656.2005.00941.x. [Google Scholar]
- Post E, Stenseth N.C. Climatic variability, plant phenology and northern ungulates. Ecology. 1999;80:1322–1339. [Google Scholar]
- Przybylo R, Sheldon B.C, Merila J. Climatic effects on breeding and morphology: evidence for phenotypic plasticity. J. Anim. Ecol. 2000;69:395–403. doi:10.1046/j.1365-2656.2000.00401.x. [Google Scholar]
- Stenseth N.C, Mysterud A, Ottersen G, Hurrell J.W, Chan K.S, Lima M. Ecological effects of climate fluctuations. Science. 2002;297:1292–1296. doi: 10.1126/science.1071281. doi:10.1126/science.1071281. [DOI] [PubMed] [Google Scholar]
- Stevenson I.R, Bryant D.M. Climate change and constraints on breeding. Nature. 2000;406:366–367. doi: 10.1038/35019151. doi:10.1038/35019151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.