Abstract
Why do females of many species mate with more than one male? One of the main hypotheses suggests that female promiscuity is an insurance mechanism against the potential detrimental effects of inbreeding. Accordingly, females should preferably mate with less related males in multiple or extrapair mating. Here we analyse paternity, relatedness among mating partners, and relatedness between parents and offspring, in the socially monogamous North American barn swallow (Hirundo rustica erythrogaster). In contrast to the inbreeding avoidance hypothesis, we found that extrapair mating partners were more related than expected by random choice, and tended to be more related than social partners. Furthermore, extrapair mating resulted in genetic parents being more related to their extrapair young than to their withinpair young. We propose a new hypothesis for extrapair mating based on kin selection theory as a possible explanation to these findings.
Keywords: extrapair mating, kin selection, inclusive fitness, optimal relatedness, Hirundo rustica
1. Introduction
Social monogamy is widespread, yet genetic monogamy is rare in a majority of the species studied so far, especially among passerine birds (reviewed in Griffith et al. 2002). The adaptive significance of male promiscuity is obvious, as it will increase the likelihood of siring a larger number of descendants. How females may benefit through mating with other males than their social partner is less obvious and much debated (e.g. Jennions & Petrie 2000; Westneat & Stewart 2003). A plethora of hypotheses have been put forward in an attempt to explain the potential benefit of female extrapair mating (e.g. reviewed in Petrie & Kempenaers 1998; Griffith et al. 2002). One of the hypotheses suggests that females mate with extrapair males to reduce the potential costs of inbreeding if socially paired with kin (Bensch et al. 1994). Certainly, a multitude of detrimental effects due to inbreeding have been revealed in a great number of studies (reviewed in Keller & Waller 2002). Currently, there has been an increasing focus on the ‘inbreeding avoidance’ hypothesis, partly due to the employment of molecular tools enabling an easier assessment of pairwise genetic relatedness (e.g. Blomqvist et al. 2002; Foerster et al. 2003).
A socially monogamous mating system imposes a constraint on the availability of social partners. Females may end up with a related or genetically similar social mate, potentially having a negative effect on reproductive success. To circumvent the constraints imposed by social monogamy and improve their fitness, females may engage in extrapair copulations with less related or genetically dissimilar males (Bensch et al. 1994).
The aim of the present study was to investigate extrapair mating in relation to relatedness in the migratory and widely abundant North American barn swallow (Hirundo rustica erythrogaster). Polymorphic microsatellite markers were used to assign paternity, obtain measures of pairwise genetic relatedness (Queller & Goodnight 1989) and indicate inbreeding costs.
2. Methods
(a) General field procedures
This study was conducted in four colonies (ranging from 6 to 68 pairs) near the Queen's University Biological Station (44°34′N, 76°19′W) in Southeast Ontario, Canada during the summers of 2003 and 2004. We captured and individually colour-marked 375 adult barn swallows, which included all except four breeding males and four breeding females, at our study sites. A small blood sample (10–150 μl) was obtained for genetic analyses. Adults were initially sexed according to phenotypic characters, but later sexed conclusively by a standard molecular method (Griffiths et al. 1998). Pairs were regularly identified during nest building, incubation and nestling provisioning. Three days after hatching, we sampled a small amount of blood (2–25 μl) from the nestlings for genetic analyses. We also collected unhatched eggs and dead young for genetic analyses. Our data document incidences of close relatives breeding within the same colony, as six out of 77 males caught for the first time in 2004 were born in the same colony in 2003, whereas no female offspring returned to their natal colony. The return rate was 53% (68/129) for adult males and 52% (54/103) for adult females.
(b) Microsatellite analyses
All but three individuals (decomposed embryos) were genotyped at nine polymorphic microsatellite markers (see electronic supplementary material). The microsatellite genotypes formed the basis for the parentage analysis, heterozygosity estimation and pairwise genetic relatedness (i.e. genetic similarity) calculations.
The nine microsatellites constituted a powerful marker set for paternity analyses, with a combined exclusion probability of 0.999996 (see table 1 in the electronic supplementary material). Heterozygosity was expressed as the proportion of heterozygous loci. Average heterozygosity in adults was 0.78±0.007 standard error of the mean (s.e.m.) (range=0.33–1.00, n=375) and in young 0.78±0.004 s.e.m. (range=0.33–1.00, n=914, excluding the three embryos with incomplete genotypes). Heterozygosity values were arcsine-transformed to approximate a normal distribution. Pairwise genetic relatedness (Queller & Goodnight 1989) was calculated using the software Relatedness v. 5.0.8. (http://www.gsoftnet.us/gsoft.html). The average relatedness value between all adult males and females (expected value=0) amounted to −0.003 (±0.0001 s.e.m. calculated by ‘jack-knifing’ over loci). An analysis of 97 broods with only withinpair offspring revealed an average relatedness value of 0.49±0.01 s.e.m. for full-siblings (expected value is 0.5). In the analyses of relatedness between parents and offspring, and between siblings, we replaced mutated offspring alleles with the most similar-sized parent allele. As some pairs were represented with more than one breeding attempt, due to either two broods within a season or multiple broods between seasons, we used ‘pair’ as the independent test unit wherever appropriate. In the analysis of heterozygosity in relation to various fitness variables, we used the first breeding attempt of a pair and an individual, respectively. The analyses of nestling survival and condition close to fledging (on day 16 post-hatch) only include data from 2003. The randomization tests were performed with Resampling Stats v. 5.0 (Resampling Stats Inc.).
3. Results and discussion
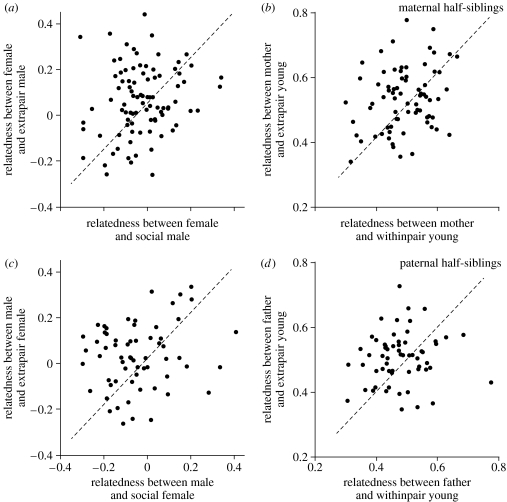
Females with extrapair paternity in their broods were more related to the extrapair sire(s) (mean relatedness±standard error of the mean (s.e.m.); 0.024±0.013, n=90) than to other males breeding in the same colony (−0.004±0.0001 s.e.m., for all pairwise combinations, randomization test; 10 000 permutations, p=0.024). Females tended to be more related to the extrapair sire(s) than to their social mate (−0.012±0.015 s.e.m., n=90) (paired t-test; t89=1.9, p=0.058; figure 1a), and females were more related to offspring sired by extrapair males than to those sired by their social mates (mean ± s.e.m.; 0.505±0.011 and 0.481±0.009, respectively; restricted maximum likelihood (REML) estimation; F1,385=7.2, n=74 maternal half-sibling dyads, p=0.008; figure 1b). Although parental relatedness predicted offspring homozygosity (Pearson correlation; r=−0.58, n=279 parent–offspring combinations, p<0.001), extrapair young were no more homozygous than their maternal half-siblings (mean ± s.e.m.; 0.78±0.01 and 0.79±0.01, respectively; REML estimation; F1,430=0.1, n=74 maternal half-sibling dyads, p=0.76). Similar patterns were found for males, whereby those that obtained extrapair fertilizations were more closely related to the extrapair female(s) than to their own social mate (paired t-test; t63=2.3, p=0.023; figure 1c). The father was also more closely related to his extrapair young than his withinpair young (REML estimation; F1,458=8.7, n=60 paternal half-sibling dyads, p=0.003, figure 1d), while the heterozygosity of the extrapair young was slightly lower than that of the withinpair young (mean ± s.e.m.; 0.77±0.01 and 0.80±0.01, respectively; REML estimation; F1,489=3.9, n=60 paternal half-sibling dyads, p=0.047). Thus, the tendency for inbred extrapair matings was evident for each sex analysed separately.
Figure 1.
Pairwise comparisons of extrapair and withinpair mate choice in barn swallows. (a) Genetic relatedness (Queller & Goodnight 1989) between female and her social and extrapair mate(s) (n=90 dyads). (b) Genetic relatedness between mother and her withinpair and extrapair offspring (n=74 dyads). (c) Genetic relatedness between male and his social and extrapair mate(s) (n=64 dyads). (d) Genetic relatedness between father and his withinpair and extrapair offspring (n=60 dyads). In all panels, the dashed lines indicate identical values, and in panels (b) and (d) the dots represent sibling group means.
If the occurrence of inbred extrapair matings was just a passive consequence of a clustering of kin in time and space, one might also expect social mates to show a bias towards inbreeding. Relatedness between social mates (mean relatedness=−0.003±0.011 s.e.m., 95% confidence interval: −0.023, 0.020, n=188) did, however, not differ from all other possible combinations of males and females breeding in the same colony (mean relatedness=−0.006±0.0001 s.e.m., 95% confidence interval: −0.0062, −0.0059; randomization test; 10 000 permutations, p=0.61). These results indicate that social pairing occurs randomly, while extrapair mating occurs non-randomly with respect to relatedness in this population of barn swallows. The observed pattern might be due to an extrapair mating preference for kin, but we are unable to determine whether the preference is exerted by males or females, or both. We are also unable to distinguish between a behavioural and a post-copulatory sperm competition/selection mechanism underlying the mating preference. Only detailed observations of copulation behaviour and studies of differential fertilization success in sperm competition can shed more light on these issues.
We lack pedigree data to confirm the relatedness estimates of mating combinations, but our data document incidences of close relatives (i.e. mothers and sons) breeding within the same colony due to male natal philopatry. The presence of relatives of opposite sex can also result if related offspring of both sexes disperse together to a non-natal colony, like the similarity in natal dispersal observed among sibling great tits (Matthysen et al. 2005).
Inbreeding can lead to decreased reproductive success and reduced individual genetic diversity of offspring (reviewed in Keller & Waller 2002), which may negatively affect their survival and fecundity (reviewed in Keller & Waller 2002). The genetic mechanism underlying inbreeding depression is reduced individual heterozygosity, leading to increased risk of recessive deleterious alleles being exposed in homozygotes or the loss of a general heterozygote advantage (reviewed in Keller & Waller 2002). We found no significant effects of individual heterozygosity on a range of parameters of reproductive success (first egg date, clutch size, egg hatching rate) and adult return rate in either sex, or on fertilization success in males (all p>0.098). In addition, relatedness between social parents had no effect on the hatching success of eggs or survival of nestlings close to fledging (Spearman rank correlations; both p>0.2). Neither did individual heterozygosity have an effect on body condition of nestlings close to fledging (general linear model; p=0.11). These results indicate low costs of inbreeding in this species. However, we emphasize that microsatellite markers might not be adequate tools to detect such costs (Pemberton 2004).
Extrapair mating has a strong effect on male reproductive success by increasing the standardized variance in realized versus apparent success eightfold in the studied population of barn swallows (Kleven et al. in press). How this mixed reproductive strategy affects female reproductive success is, however, unclear. Can the observed pattern of extrapair mating between relatives be adaptive? Hamilton's theory of kin selection (Hamilton 1964; Maynard Smith 1964; Dawkins 1979) explains how genes for altruistic behaviour can spread if they enhance the reproductive success of relatives. Inbreeding might be viewed as a kin-selected behaviour because it increases relatedness to offspring through shared genes transmitted by relatives, but at a potential cost of reduced offspring fitness (reviewed in Keller & Waller 2002). A necessary condition to obtain inclusive fitness benefits is, however, that the inbreeding event does not preclude additional matings for the mating partner (Dawkins 1979; Waser et al. 1986). This holds true for females in certain polygynous systems, in which male mating success is not limited by previous matings (Smith 1979; Lehmann & Perrin 2003). The same condition applies to extrapair mating systems, common in socially monogamous birds (Petrie & Kempenaers 1998; Griffith et al. 2002). Here, extrapair copulations incur minimal costs for the male, and typically have an additive effect on male reproductive success (number of offspring increases) (Webster et al. 1995; Kleven et al. in press). Females can, therefore, increase the reproductive success of male relatives by choosing them for extrapair copulation, but the cost of inbreeding must not exceed the fitness increment of the male partner, discounted by the coefficient of relatedness. The trade-off between the cost and benefit of inbreeding may then determine an optimal extrapair mate preference based on relatedness, according to Hamilton's rule (Hamilton 1964). From the male perspective, inbreeding is unlikely to increase the indirect component of inclusive fitness because extrapair fertilizations have no additive effect on female reproductive success. Likewise, kin selection will not favour inbreeding for either sex in a strictly monogamous system (Dawkins 1979). A prediction of this ‘optimal relatedness’ hypothesis is that female mate preferences in extrapair mating systems should vary among species or populations along an inbreeding–outbreeding continuum, depending on the cost of inbreeding.
A combination of male and female factors are probably required to fully explain the adaptive significance of extrapair paternity in most species (Westneat & Stewart 2003). The novel result of our study is the documentation of extrapair mating between relatives. Although the weak inbreeding committed by barn swallows may seem consistent with kin-selection theory, it is still premature to conclude whether the bias in relatedness is the outcome of a female extrapair mate preference for kin. Anyhow, the inclusive fitness benefit of inbreeding has been overlooked in previous theories of female extrapair copulation behaviour (e.g. Petrie & Kempenaers 1998; Griffith et al. 2002) and recent reviews of female multiple mating (e.g. Jennions & Petrie 2000; Tregenza & Wedell 2000). Hence, the ‘optimal relatedness’ hypothesis may provide a new conceptual framework for the study of the diversity of extrapair mating behaviour in birds.
Acknowledgments
We are grateful to the Ontario Ministry of Transportation for allowing us to work in their sand sheds, to the Trainor family for kindly giving us access to their property and to Floyd Connor, Rachel Fraser, Rod Green, Rasa Izadnegahdar, Frank Phelan, Mary Stapleton and Sylvia Wood for all their help and support during the field work. We thank Trond Øigarden for assistance with the molecular sex analyses, and Thomas Borge, Peter O. Dunn, Frode Fossøy, Lars Erik Johannessen, Arild Johnsen and Tore Slagsvold for constructive comments on a previous version of the manuscript. The study was supported by a doctoral fellowship grant (to O. K.) and a research grant (to J. T. L.) from the Research Council of Norway, and a grant from NSERC, Canada (to R. J. R.). Field work was approved by the Canadian Wildlife Service (permit no: CA 0121) and Queen's University Animal Care Committee (protocol no: RobertsonRJ-2003-03-0r).
Supplementary Material
References
- Bensch S, Hasselquist D, Vonschantz T. Genetic similarity between parents predicts hatching failure—nonincestuous inbreeding in the great reed warbler. Evolution. 1994;48:317–326. doi: 10.1111/j.1558-5646.1994.tb01314.x. [DOI] [PubMed] [Google Scholar]
- Blomqvist D, Andersson M, Kupper C, Cuthill I.C, Kis J, Lanctot R.B, Sandercock B.K, Szekely T, Wallander J, Kempenaers B. Genetic similarity between mates and extra-pair parentage in three species of shorebirds. Nature. 2002;419:613–615. doi: 10.1038/nature01104. doi:10.1038/nature01104 [DOI] [PubMed] [Google Scholar]
- Dawkins R. Twelve misunderstandings of kin selection. Z. Tierpsychol. 1979;51:184–200. [Google Scholar]
- Foerster K, Delhey K, Johnsen A, Lifjeld J.T, Kempenaers B. Females increase offspring heterozygosity and fitness through extra-pair matings. Nature. 2003;425:714–717. doi: 10.1038/nature01969. doi:10.1038/nature01969 [DOI] [PubMed] [Google Scholar]
- Griffith S.C, Owens I.P.F, Thuman K.A. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. doi:10.1046/j.1365-294X.2002.01613.x [DOI] [PubMed] [Google Scholar]
- Griffiths R, Double M.C, Orr K, Dawson R.J.G. A DNA test to sex most birds. Mol. Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. doi:10.1046/j.1365-294x.1998.00389.x [DOI] [PubMed] [Google Scholar]
- Hamilton W.D. The genetical evolution of social behavior, I and II. J. Theor. Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. doi:10.1016/0022-5193(64)90038-4 [DOI] [PubMed] [Google Scholar]
- Jennions M.D, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 2000;75:21–64. doi: 10.1017/s0006323199005423. doi:10.1017/S0006323199005423 [DOI] [PubMed] [Google Scholar]
- Keller L.F, Waller D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002;17:230–241. doi:10.1016/S0169-5347(02)02489-8 [Google Scholar]
- Kleven, O., Jacobsen, F., Izadnegahdar, R., Robertson, R. J. & Lifjeld, J. T. In press. Male tail streamer length predicts fertilization success in the North American barn swallow (Hirundo rustica erythrogaster). Behav. Ecol. Sociobiol
- Lehmann L, Perrin N. Inbreeding avoidance through kin recognition: choosy females boost male dispersal. Am. Nat. 2003;162:638–652. doi: 10.1086/378823. doi:10.1086/378823 [DOI] [PubMed] [Google Scholar]
- Matthysen E, Van de Casteele T, Adriaensen F. Do sibling tits (Parus major, P. caeruleus) disperse over similar distances and in similar directions? Oecologia. 2005;143:301–307. doi: 10.1007/s00442-004-1760-7. doi:10.1007/s00442-004-1760-7 [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Group selection and kin selection. Nature. 1964;201:1145–1147. [Google Scholar]
- Pemberton J. Measuring inbreeding depression in the wild: the old ways are the best. Trends Ecol. Evol. 2004;19:613–615. doi: 10.1016/j.tree.2004.09.010. doi:10.1016/j.tree.2004.09.010 [DOI] [PubMed] [Google Scholar]
- Petrie M, Kempenaers B. Extra-pair paternity in birds: explaining variation between species and populations. Trends Ecol. Evol. 1998;13:52–58. doi: 10.1016/s0169-5347(97)01232-9. doi:10.1016/S0169-5347(97)01232-9 [DOI] [PubMed] [Google Scholar]
- Queller D.C, Goodnight K.F. Estimating relatedness using genetic markers. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. [DOI] [PubMed] [Google Scholar]
- Smith R.H. On selection for inbreeding in polygynous animals. Heredity. 1979;43:205–211. [Google Scholar]
- Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage: invited review. Mol. Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. doi:10.1046/j.1365-294x.2000.00964.x [DOI] [PubMed] [Google Scholar]
- Waser P.M, Austad S.N, Keane B. When should animals tolerate inbreeding. Am. Nat. 1986;128:529–537. doi:10.1086/284585 [Google Scholar]
- Webster M.S, Pruett-Jones S, Westneat D.F, Arnold S.J. Measuring the effects of pairing success, extra-pair copulations and mate quality on the opportunity for sexual selection. Evolution. 1995;49:1147–1157. doi: 10.1111/j.1558-5646.1995.tb04441.x. [DOI] [PubMed] [Google Scholar]
- Westneat D.F, Stewart I.R.K. Extra-pair paternity in birds: causes, correlates and conflict. Annu. Rev. Ecol. Evol. Syst. 2003;34:365–396. doi:10.1146/annurev.ecolsys.34.011802.132439 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.