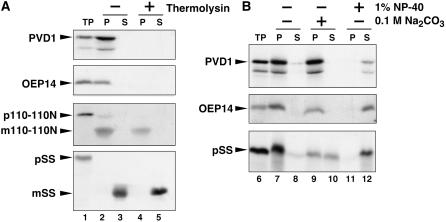
Figure 7.
In Vitro Chloroplast Import, Protease Protection, and Fractionation Assay of PDV1.
35S-labeled PDV1, the integral outer envelope protein OEP14, the precursor of truncated inner envelope protein Tic110-110N (110-110N), and the precursor of the stroma-localized small subunit of ribulose-1,5-bis-phosphate carboxylase/oxygenase (SS) were synthesized in vitro. In vitro–translated proteins were incubated with isolated pea chloroplasts in the presence of 4.0 mM Mg-ATP for 30 min at room temperature. Chloroplasts were recovered by sedimentation through 40% (v/v) Percoll. p and m indicate precursor and mature proteins for 110-110N and SS, respectively.
(A) The recovered intact chloroplasts were incubated without (−; lanes 2 and 3) or with (+; lanes 4 and 5) thermolysin for 30 min at 4°C. Intact chloroplasts were again recovered by centrifugation through 40% (v/v) Percoll and fractionated into total membrane (P; lanes 2 and 4) and soluble (S; lanes 3 and 5) fractions. TP represents 10% of translated product added to a single import assay (lane 1).
(B) The recovered intact chloroplasts were lysed and fractionated into total membrane (P) and soluble (S) fractions (lanes 7 and 8). A portion of the membranes recovered in lane 7 for each import reaction was subsequently extracted with 0.1 M sodium carbonate, pH 11.5 (lanes 9 and 10), or with 1% Nonidet P-40 (lanes 11 and 12) for 1 h on ice. After the treatments, membranes (P) and extractable soluble proteins (S) were recovered and analyzed by SDS-PAGE and fluorography. TP represents 10% of translated product added to a single import assay (lane 6).