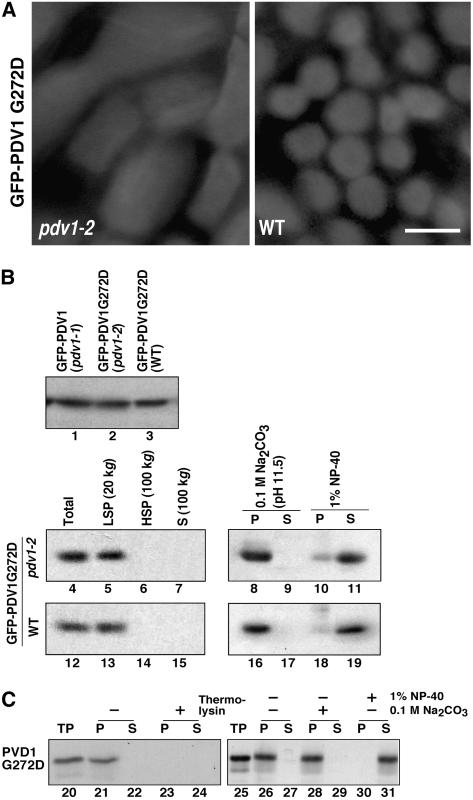
Figure 8.
Effect of the C-Terminal Mutation of PDV1 (pdv1-2) on PDV1 Localization.
(A) GFP-PDV1G272D was expressed in pdv1-2 (left) and wild-type (right) plants. GFP signals in mesophyll cells of young leaves were observed by fluorescence microscopy. Bar = 5 μm.
(B) Expression levels of GFP-PDV1 in pdv1-1 plants (lane 1), which showed ring structures, and of GFP-PDV1G272D in pdv1-2 (lane 2) and wild-type (lane 3) plants were compared. Homogenates of flower buds were analyzed by immunoblotting using anti-GFP antibody. The total homogenates (lanes 4 and 12) were fractionated into the low-speed pellet (LSP; lanes 5 and 13), the high-speed pellet (HSP; lanes 6 and 14), and the supernatant (S; lanes 7 and 15) fractions. The low-speed pellet fraction was treated with 0.1 M sodium carbonate (lanes 8, 9, 16, and 17) or 1% Nonidet P-40 (NP-40; lanes 10, 11, 18, and 19), and then soluble (S; lanes 9, 11, 17, and 19) and insoluble (P; lanes 8, 10, 16, and 18) fractions were separated. Homogenates from pdv1-2 (lanes 4 to 11) and wild-type (lanes 12 to 19) plants expressing GFP-PDV1G272D were fractionated by the same methods as described for Figure 3.
(C) In vitro chloroplast import, protease protection, and fractionation assay of PDV1G272D. 35S-labeled PDV1G272D was synthesized in vitro. The translation product was incubated with isolated chloroplasts, and assays were performed as described for Figure 7. TP represents 10% of translated product added to a single import assay (lanes 20 and 25).