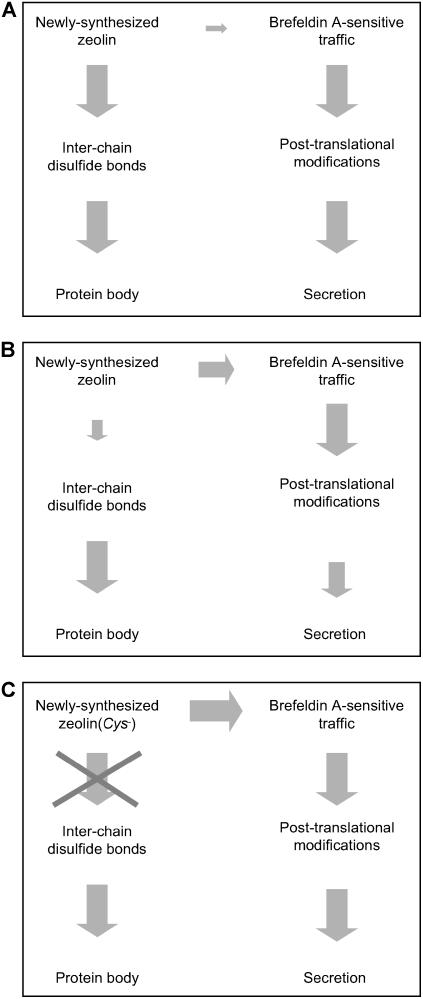
Figure 10.
Model Illustrating the Influence of Disulfide Bonds on Zeolin Destiny.
The size of the gray arrows is roughly proportional to the efficiency of each step, independent of that of the preceding or competing step. Of course, an inefficient or impossible step (small arrows or crossed arrow) becomes limiting for a pathway even if the subsequent steps are efficient or are unaffected by a given treatment (large arrows), but such a cascade of events is not illustrated here because we wish to emphasize key effects.
(A) Destiny of zeolin in normal conditions. The formation of disulfide bonds is very efficient and leads to PB formation, almost completely avoiding traffic that would lead to processing and secretion.
(B) Destiny of zeolin in cells treated with 2-ME. General traffic and secretion are negatively affected, probably at multiple steps, but not fully blocked. Disulfide bond formation of zeolin is also inhibited, allowing the protein to enter traffic.
(C) Destiny of zeolin(Cys−). The formation of disulfide bonds is impossible; therefore, the protein enters traffic with high efficiency.