Figure 2.
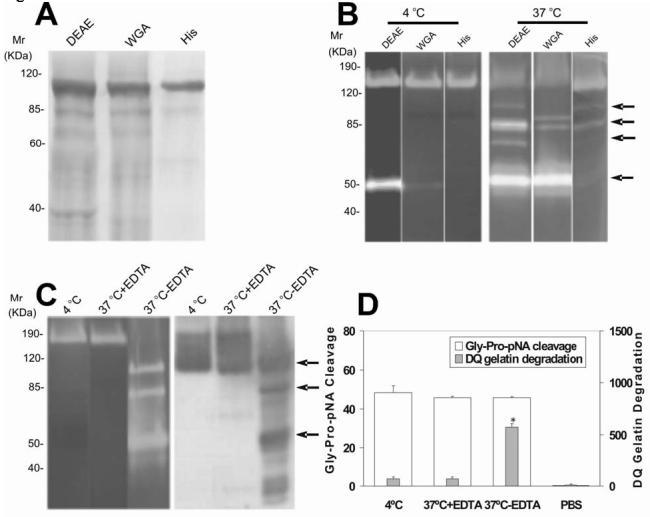
Purification and proteolytic activities of r-seprase. A, SDS PAGE analysis on purified r-seprase. The r-seprase samples indicated were increasingly and sequentially purified by the DEAE Sepharose column (DEAE), the WGA affinity chromatography column (WGA) and the His·Bind Resin column (His) from the culture medium conditioned by pE15 transfected 293-EBNA cells. The samples were then heated to 100 °C for 3 min in SDS sampling buffer and separated by SDS PAGE. The gel was stained with Coomassie Brilliant Blue. Each lane corresponds to the dissociated monomers of r-seprase present in 10-mL of original culture medium. B, Increased gelatinase activity of purified r-seprase incubated at 37 °C. R-seprase samples were purified as above, but not heated, were incubated at 4 °C (left panel) or 37 °C (right panel) overnight, and then subjected to gelatin zymography in 5 mM EDTA conditions. The top two arrows show the 100-85 kDa gelatinases and the lower two arrows indicate the 70-50 kDa gelatinases that were present only in the less purified fractions, which were potentially exposed to other proteases. Each lane corresponds to the r-seprase present in 10-mL of original culture medium. C, Activation of the 160-kDa r-seprase into 100-85 kDa and 70-50 kDa gelatinases (arrows) by EDTA sensitive protease. R-seprase was enriched by WGA-affinity chromatography column and incubated at 4 °C or 37 °C for 1 day in the presence or absence of 5 mM EDTA (indicated by 4 °C, 37°C+EDTA and 37°C-EDTA, respectively), and then subjected to gelatin zymography in 5 mM EDTA conditions and Western immunoblotting using mAb E97 (against seprase subunits and shortened forms that were denatured by Western immunoblotting transfer buffer). The top two arrows show the 100-85 kDa gelatinases and the lowest arrow indicates the 50 kDa gelatinases. D, Activation of r-seprase increases gelatinase activity, but not DP activity. R-seprase was enriched by a WGA-affinity chromatography column and incubated at 4 °C or 37 °C for 1 day in the presence or absence of 5 mM EDTA (indicated by 4 °C, 37°C+EDTA and 37°C-EDTA, respectively), and then subjected to the soluble enzymatic assays as described (15). PBS was used as a negative control in the soluble enzymatic assay. The values shown are the mean ± SD. The symbol * above the bar is used to indicate a significant increase (p<0.001) in gelatinase activity of truncated seprase.
