Figure 3.
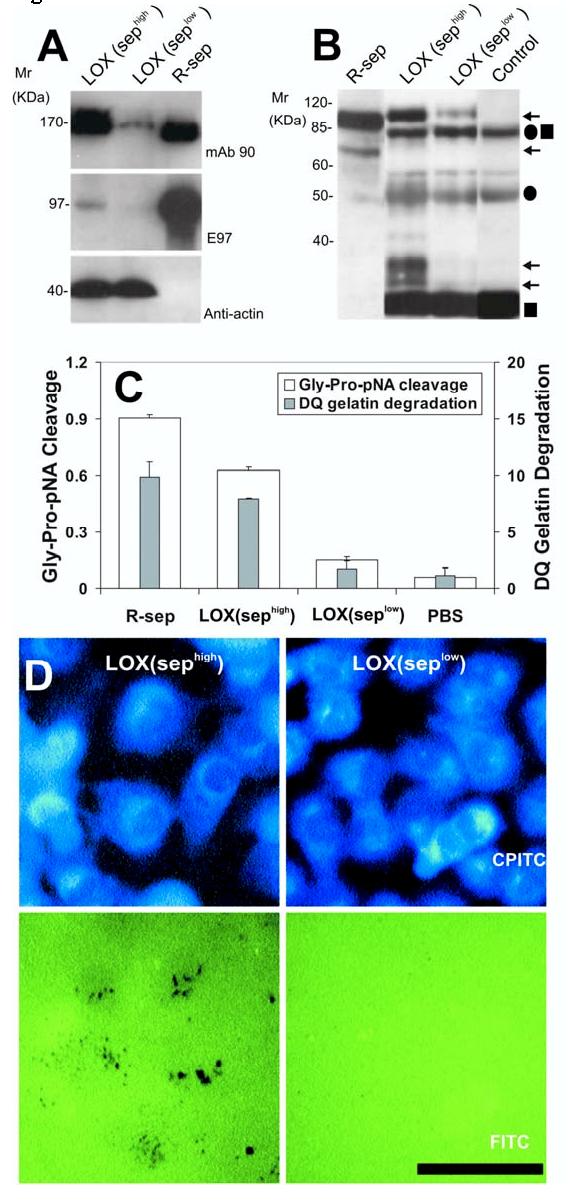
Expression and activity of n-seprase in LOX melanoma cell lines and tumor tissues from a xenograft model. A, N-seprase in LOX cell lines, sephigh and seplow. LOX cells transfected with the control pGUS vector express high levels of seprase, whereas transfection with the pGUS-SEP 1384 vector utilized RNA interference (RNAi) creates a cell line with limited seprase expression. Cell lysates of the two LOX cell lines, sephigh and seplow, were subjected to SDS-PAGE followed by Western immunoblotting under non-boiling (for mAb 90) and boiling conditions (for mAb E97 and mAb anti-actin). The lane labeled r-sep is the positive control r-seprase; the band labeled anti-actin serves as a protein loading control. B, N-seprase in experimental tumors created from the two LOX cell lines, sephigh and seplow. Tumors were lysed with EDTA-containing buffer. N-seprase was isolated from tumor tissue lysates by immunoprecipitation using mAb D8-D28-D43 conjugated beads that specifically target seprase antigen. The beads-antigen complexes were boiled in SDS-PAGE loading buffer to release seprase polypeptides, and separated via SDS-PAGE. Subsequent Western immunoblotting using rat mAb E97 revealed bands corresponding to n-seprase polypeptides of 97-, 65-, 35- and 25-kDa (arrows). Antibody IgG fragments released from the beads, including linked heavy and light chains (•■), heavy chain alone (•) and light chain alone (■), were also detected. R-seprase (r-sep) was used as a positive control. The negative control (control) was a protein sample derived from the antibody beads that were not mixed with tumor lysates. C, Seprase specific DP and gelatinase activity in experimental tumors created from the two LOX cell lines, sephigh and seplow. Tumor tissue lysates were applied to plates coated with mAb D8 to specifically capture active seprase forms. The soluble enzymatic assays were conducted in the presence of 5 mM EDTA to inhibit any metalloprotease activity. The experiment was performed in triplicate and the values are the mean ± SD. A significant increase (p<0.001) in seprase activity compared to the seprase knockdown tumors is indicated by the * above the bar. D, Degradation/invasion of fibronectin-coated cross-linked gelatin substrata comparing two conditions of LOX cells, LOX (sephigh) and LOX (seplow). LOX cells transfected with pGUS and pGUS-sep1384, respectively, were cultured on FITC-fibronectin-coated crosslinked gelatin films. After fixation and Phalloidin-CPITC staining, cells were visualized by epifluorescence microscopy of CPITC to visualize cell location, and the same fields were then photographed by epifluorescence microscopy of FITC to visualize the sites of matrix degradation left behind the migrating cells which are shown by dark spots on the green background. Bar, 50 μm.
