Figure 5.
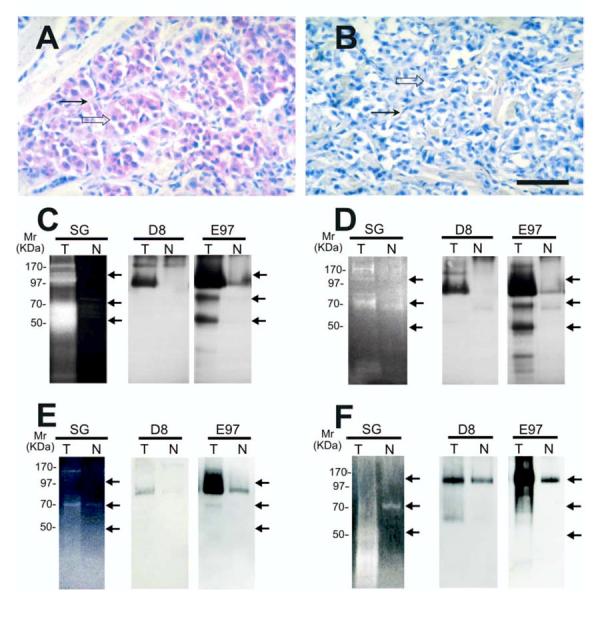
Gelatinase activity and localization of n-seprase in other human tumors. A and B, Immunohistochemical localization of n-seprase in human malignant melanoma. Formalin-fixed, paraffin-embedded human malignant melanoma tissue was sectioned and stained using rat mAb D8 (A) and the control rat mAb (B). The slides were then incubated with biotin-conjugated goat anti-rat antibody (Jackson Immunoresearch, West Grove, PA), stained with strepavidin/alkaline phosphatase conjugate (Jackson Immunoresearch), followed by Fast red chromogen in napthol phosphate reconstitution buffer (Biogenex) for the color development and counterstained with hematoxylin. Note that seprase positive regions of melanoma cells (open arrow) and stromal cells (arrow) are stained red. Bar, 50 μm. C -F, Detection of n-seprase activated forms of different size in invasive carcinomas of the colon (C), stomach (D) and breast (E), and in malignant melanoma (F). Paired tumor (T) and adjacent normal (N) tissues from the same patient were lysed with EDTA-containing lysis buffer. Glycosylated protein captured by the WGA column from the tissue lysates were analyzed in parallel by gelatin zymography (SG) and immunoblotting (D8 and E97). The protein samples were loaded under non-boiling, non-reducing conditions to retain gelatinase activity of seprase active forms. The gelatinase activity of serine-type gelatinases (SG) was detected by incubating the zymogram with 5 mM EDTA to inhibit metalloprotease activity as described (15). Note that two major groups of active s-seprase forms (100-85 kDa and 70-50 kDa indicated by arrows) were found by parallel analysis of gelatin zymography and Western immunoblotting. In immunoblotting, mAb D8 recognizes mainly seprase 170-kDa dimeric form and 97-kDa dissociated monomeric form, while mAb E97 recognizes all seprase forms, including seprase 170-kDa dimeric form, 97-kDa monomeric subunit, activated forms of 100-85 kDa and 70-50 kDa (arrows), and other inactive shortened forms.
