Figure 6.
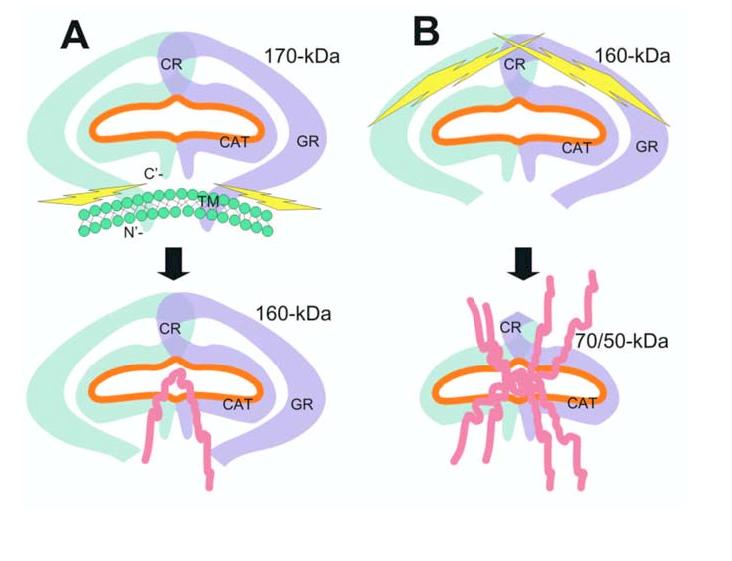
Schematic illustration of a potential seprase activation process. A, Release of membrane-bound n-seprase. The dimeric form of n-seprase (170-kDa) is bound to the cell membrane by the N-terminal stalk region and creates a catalytic pocket (orange line). Likely there is more than one overlapping region that contributes to the dimerization of this protein. The membrane blocks access to the active pocket when the 3-D structure of the dimer is considered. Activating proteases cleave the dimer at the stalk region and release it from the membrane, thereby creating a soluble form like r-seprase (160-kDa). The separation of the protein from the membrane reduces steric hindrance and increases accessibility to the catalytic pocket from below. B, Further N-terminal truncation of seprase and increase of gelatinase activity. Release of N-terminal peptides from the 160-kDa seprase into the 70-/50-kDa form extensively reduces steric hindrance and permits even greater accessibility of substrates to the catalytic site. The amino acid terminus and domains of seprase are indicated: C’, carboxyl terminus; N’, amino terminus; TM, transmembrane domain, amino acids 7-26; GR, glycosylation rich region, amino acids 49-314; CR, cysteine rich domain, amino acids 305-466; CAT, catalytic domain, amino acids 500-760.
