Abstract
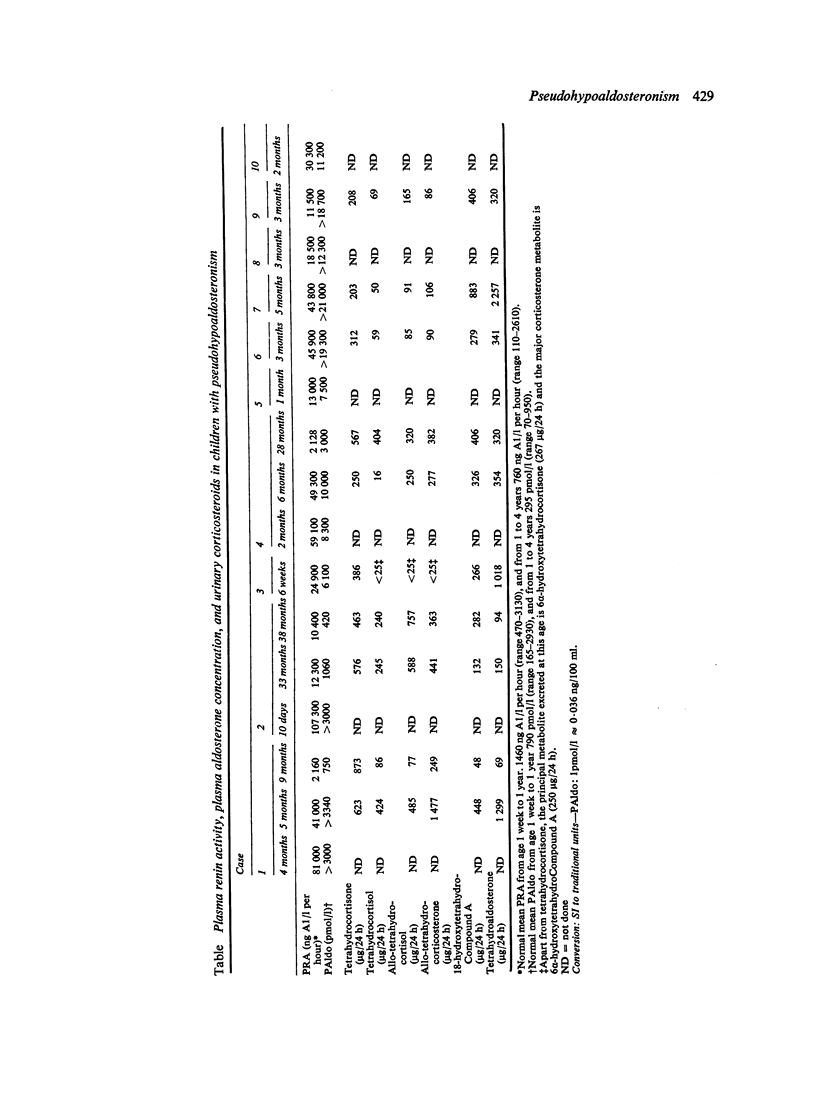
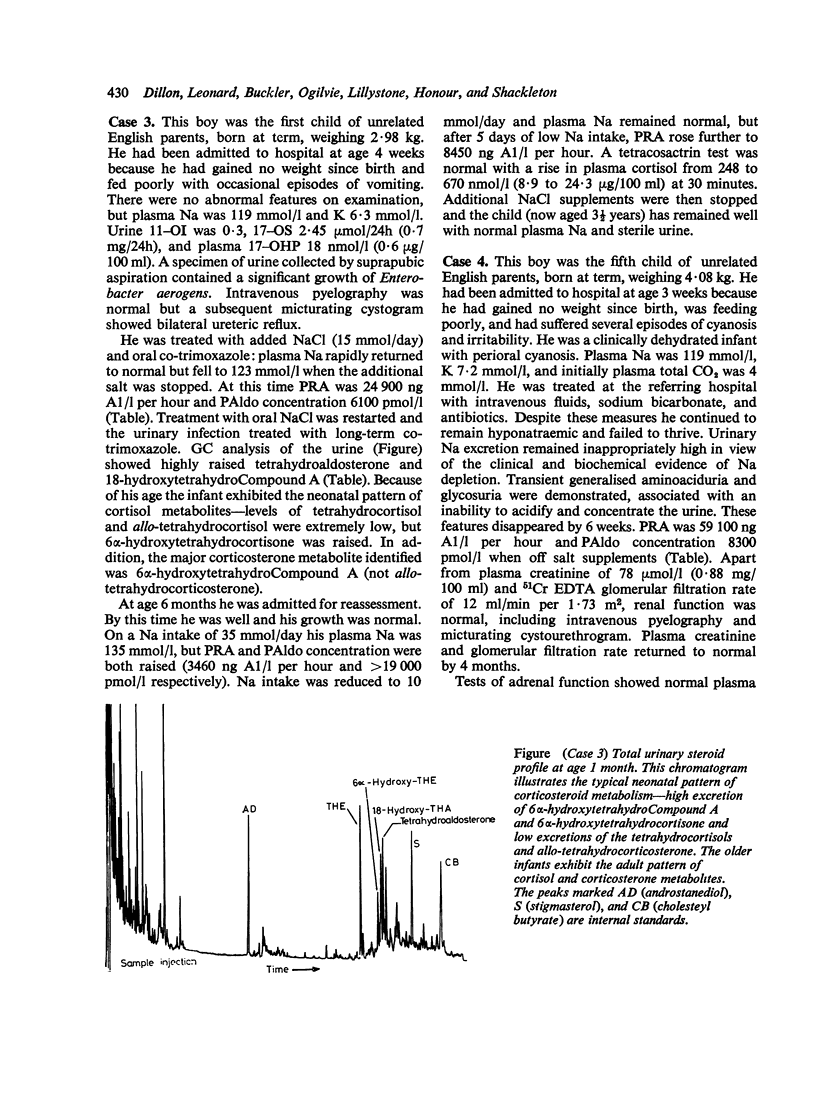
10 infants are described with pseudohypoaldosteronism, 5 in detail and a further 5 briefly. They all presented with hyperkalaemia, urinary salt-wasting disease, and ostensibly normal renal and adrenocortical function. Diagnosis was established by demonstrating the greatly increased values of plasma renin activity and plasma aldosterone concentration, plus the increased excretion of aldosterone and its metabolites on gas chromatographic and mass spectrometric analyses of urine. The children were treated with sodium chloride supplements, up to 60 mmol/day, but by the time most of the infants were about a year old these could be stopped. Exogenous mineralocorticoids were without effect in those to whom they were administered. The precise aetiology of the condition remains conjectural; lack of renal tubular response to aldosterone seems probable. Pseudohypoaldosteronism may be more common than has been thought and new techniques for investigating salt-wasting disorders may show its true incidence.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes N. D., Atherden S. M. Diagnosis of congenital adrenal hyperplasia by measurement of plasma 17-hydroxyprogesterone. Arch Dis Child. 1972 Feb;47(251):62–65. doi: 10.1136/adc.47.251.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes N. D., Joseph J. M., Atherden S. M., Clayton B. E. Functional tests of adrenal axis in children with measurement of plasma cortisol by competitive protein binding. Arch Dis Child. 1972 Feb;47(251):66–73. doi: 10.1136/adc.47.251.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthe P. h., Thal V. K., Bouissou F., Rochiccioli P., Voight J. J., Bayard F. A propos d'un cas de pseudohypoaldosteronisme (etude du taux de secretion d'aldosterone) Arch Fr Pediatr. 1974 Dec;31(10):973–984. [PubMed] [Google Scholar]
- Bierich J. R., Schmidt U. Tubular Na, K-ATPase deficiency, the cause of the congenital renal salt-losing syndrome. Eur J Pediatr. 1976 Jan 2;121(2):81–87. doi: 10.1007/BF00443063. [DOI] [PubMed] [Google Scholar]
- CHEEK D. B., PERRY J. W. A salt wasting syndrome in infancy. Arch Dis Child. 1958 Jun;33(169):252–256. doi: 10.1136/adc.33.169.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON B. E., EDWARDS R. W., RENWICK A. G. Adrenal function in children. Arch Dis Child. 1963 Feb;38:49–53. doi: 10.1136/adc.38.197.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORBEEL L. [Saline diabetes in the infant without adrenal insufficiency]. Pediatrie. 1963;18:557–562. [PubMed] [Google Scholar]
- Clayton B. E., Edwards R. W., Makin H. L. Congenital adrenal hyperplasia and other conditions associated with a raised urinary steroid 11-oxygenation index. J Endocrinol. 1971 Jun;50(2):251–265. doi: 10.1677/joe.0.0500251. [DOI] [PubMed] [Google Scholar]
- DONNELL G. N., LITMAN N., ROLDAN M. Pseudohypo-adrenalocorticism; renal sodium loss, hyponatremia, and hyperkalemia due to a renal tubular insensitivity to mineralocorticoids. AMA J Dis Child. 1959 Jun;97(6):813–828. [PubMed] [Google Scholar]
- Dillon M. J. Measurement of plasma renin activity by semi-micro radioimmunoassay of generated angiotensin I. J Clin Pathol. 1975 Aug;28(8):625–630. doi: 10.1136/jcp.28.8.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon M. J., Ryness J. M. Plasma renin activity and aldosterone concentration in children. Br Med J. 1975 Nov 8;4(5992):316–319. doi: 10.1136/bmj.4.5992.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honour J. W., Shackleton C. H. Mass spectrometric analysis for tetrahydroaldosterone. J Steroid Biochem. 1977 Apr;8(4):299–305. doi: 10.1016/0022-4731(77)90023-1. [DOI] [PubMed] [Google Scholar]
- LELONG M., ALAGILLE D., PHILIPPE A., GENTIL C., GABILAN J. C. [Saline diabetes caused by congenital insensitivity of the tubule to aldosterone: "pseudo-hypoadrenocorticism"]. Rev Fr Etud Clin Biol. 1960 Jun-Jul;5:558–565. [PubMed] [Google Scholar]
- Lauras B., Ravussin J. J., David M., Freycon F., Jeune M. Pseudo-hypoaldosteronisme chez l'enfant. A propos de quatre observations dont deux concernant des frères. Pediatrie. 1978 Mar;33(2):119–135. [PubMed] [Google Scholar]
- Milla P. J., Trompeter R., Dillon M. J., Robins D., Shackleton C. Salt-losing syndrome in 2 infants with defective 18-dehydrogenation in aldosterone biosynthesis. Arch Dis Child. 1977 Jul;52(7):580–586. doi: 10.1136/adc.52.7.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. E. Some studies of the protein-binding of steroids and their application to the routine micro and ultramicro measurement of various steroids in body fluids by competitive protein-binding radioassay. J Clin Endocrinol Metab. 1967 Jul;27(7):973–990. doi: 10.1210/jcem-27-7-973. [DOI] [PubMed] [Google Scholar]
- PROUT M., SNAITH A. H. Urinary excretion of 17-ketosteroids in children. Arch Dis Child. 1958 Aug;33(170):301–304. doi: 10.1136/adc.33.170.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S., Giese J., Kappelgaard A. M., Lund H. T., Lund J. O., Nielsen M. D., Thomsen A. C. Pseudohypoaldosteronism. Clinical, biochemical and morphological studies in a long-term follow-up. Acta Paediatr Scand. 1978 Mar;67(2):255–261. doi: 10.1111/j.1651-2227.1978.tb16314.x. [DOI] [PubMed] [Google Scholar]
- Pham-Huu-Trung, Piussan C., Rodary C., Legrand S., Attal C., Mozziconacci P. Etude du taux de sécrétion de l'aldosterone et de l'activité de la rénine plasmatique d'un cas de pseudo-hypoaldosteronisme. Arch Fr Pediatr. 1970 Jun-Jul;27(6):603–615. [PubMed] [Google Scholar]
- Polonovski C., Zittoun R., Mary F. Hypocorticisme global. Hypoaldosteronisme et pseudo-hypoaldosteronisme du nourrisson. Trois observations. Arch Fr Pediatr. 1965 Nov;22(9):1061–1086. [PubMed] [Google Scholar]
- Postel-Vinay M. C., Alberti G. M., Ricour C., Limal J. M., Rappaport R., Royer P. Pseudohypoaldosteronism: persistence of hyperaldosteronism and evidence for renal tubular and intestinal responsiveness to endogenous aldosterone. J Clin Endocrinol Metab. 1974 Dec;39(6):1038–1044. doi: 10.1210/jcem-39-6-1038. [DOI] [PubMed] [Google Scholar]
- Proesmans W., Geussens H., Corbeel L., Eeckels R. Pseudohypoaldosteronism. Am J Dis Child. 1973 Oct;126(4):510–516. doi: 10.1001/archpedi.1973.02110190418013. [DOI] [PubMed] [Google Scholar]
- RAINE D. N., ROY J. A salt-losing syndrome in infancy. Pseudo-hypoadrenocorticalism. Arch Dis Child. 1962 Oct;37:548–556. doi: 10.1136/adc.37.195.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROYER P., BONNETTE J., MATHIEU H., GABILAN J. C., KLUTCHKO G., ZITTOUN R. PSEUDO-HYPOALDOST'ERONISME. Ann Pediatr (Paris) 1963 Dec 2;10:596–605. [PubMed] [Google Scholar]
- Rampini S., Furrer J., Keller H. P., Bucher H., Zachmann M. Congenital pseudohypoaldosteronism: case report and review. Effect of indomethacin during sodium chloride depletion. Helv Paediatr Acta. 1978 Jun;33(2):153–167. [PubMed] [Google Scholar]
- Rees L. H., Cook D. M., Kendall J. W., Allen C. F., Kramer R. M., Ratcliffe J. G., Knight R. A. A radioimmunoassay for rat plasma ACTH. Endocrinology. 1971 Jul;89(1):254–261. doi: 10.1210/endo-89-1-254. [DOI] [PubMed] [Google Scholar]
- Roy C. Pseudohypoaldosteronisme familial (a propos de 5 cas) Arch Fr Pediatr. 1977 Jan;34(1):37–54. [PubMed] [Google Scholar]
- Rösler A., Rabinowitz D., Theodor R., Ramirez L. C., Ulick S. The nature of the defect in a salt-wasting disorder in Jews of Iran. J Clin Endocrinol Metab. 1977 Feb;44(2):279–291. doi: 10.1210/jcem-44-2-279. [DOI] [PubMed] [Google Scholar]
- Rösler A., Theodor R., Boichis H., Gerty R., Ulick S., Alagem M., Tabachinik E., Cohen B., Rabinowitz D. Metabolic responses to the administration of angiotensin II, K and ACTH in two salt-wasting syndromes. J Clin Endocrinol Metab. 1977 Feb;44(2):292–301. doi: 10.1210/jcem-44-2-292. [DOI] [PubMed] [Google Scholar]
- Rösler A., Theodor R., Gazit E., Biochis H., Rabinowitz D. Salt wasting, raised plasma-renin activity, and normal or high plasma-aldosterone: a form of pseudohypoaldosteronism. Lancet. 1973 May 5;1(7810):959–962. doi: 10.1016/s0140-6736(73)91599-7. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Schmid J., Schmid H., Dubach U. C. Sodium- and potassium-activated ATPase. A possible target of aldosterone. J Clin Invest. 1975 Mar;55(3):655–660. doi: 10.1172/JCI107973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton C. H., Honour J. W., Dillon M., Milla P. Multicomponent gas chromatographic analysis of urinary steroids excreted by an infant with a defect in aldosterone biosynthesis. Acta Endocrinol (Copenh) 1976 Apr;81(4):762–773. doi: 10.1530/acta.0.0810762. [DOI] [PubMed] [Google Scholar]
- Shackleton C. H., Honour J. W. Identification and measurement of 18-hydroxycorticosterone metabolites by gas chromatography-mass spectrometry. J Steroid Biochem. 1977 Mar;8(3):199–203. doi: 10.1016/0022-4731(77)90051-6. [DOI] [PubMed] [Google Scholar]
- Shackleton C. H., Snodgrass G. H. Steroid excretion by an infant with an unusual salt-losing syndrome: a gas chromatographic-mass spectrometric study. Ann Clin Biochem. 1974 May;11(3):91–99. doi: 10.1177/000456327401100134. [DOI] [PubMed] [Google Scholar]
- Strickland A. L., Kotchen T. A. A study of the renin-aldosterone system in congenital adrenal hyperplasia. J Pediatr. 1972 Nov;81(5):962–969. doi: 10.1016/s0022-3476(72)80550-x. [DOI] [PubMed] [Google Scholar]


