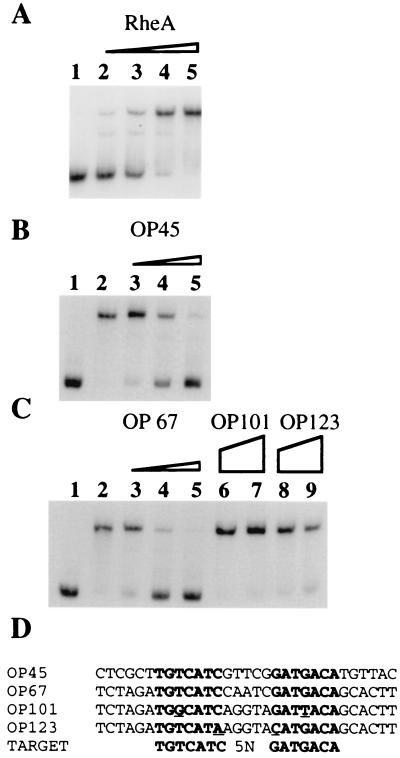
Figure 3.
In vitro analysis of RheA binding to a S. albus DNA fragment containing the hsp18 and rheA promoters. Gel retardation assays were performed using a 270-bp radiolabeled DNA fragment corresponding to the rheA-hsp18 promoters. Gels were run at room temperature. (A) ≈0.05 pmol of labeled DNA fragment was incubated with various amounts of purified RheA: lane 1, no protein; lane 2, 21.6 fmol; lane 3, 43.2 fmol; lane 4, 86.5 fmol; lane 5, 130 fmol. (B) Each lane contains ≈0.05 pmol of the labeled 270-bp fragment, 130 fmol of purified RheA (except lane 1), and one of a series of amounts of an unlabeled oligonucleotide competitor. Lane 1, no protein; lane 2, no competitor; lane 3, 0.06 pmol of OP45; lane 4, 0.12 pmol of OP45; lane 5, 0.24 pmol of OP45. (C) Each lane contains ≈0.05 pmol of the labeled 270-bp fragment, 130 fmol of purified RheA (except lane 1) and one of a series of amounts of an unlabeled oligonucleotide competitor. Lane 1, no protein; lane 2, no competitor; lane 3, 0.06 pmol of OP67; lane 4, 0.12 pmol of OP67; lane 5, 0.24 pmol of OP67; lane 6, 0.5 pmol of OP101; lane 7, 1.5 pmol of OP101; lane 8, 0.5 pmol of OP123; lane 9, 1.5 pmol of OP123. (D) Sequences of oligonucleotides used in the unlabeled competitor chase experiments.