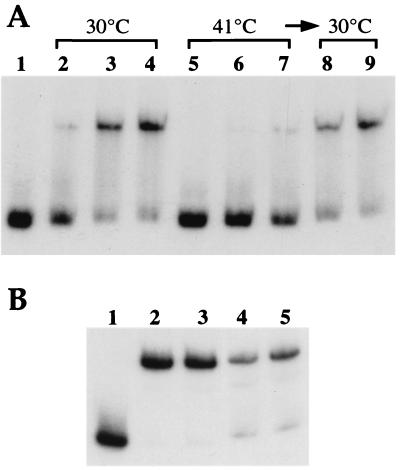
Figure 5.
Gel mobility-shift assays showing the loss of RheA DNA-binding capacity at high temperature and its recovery at low temperature. Gel retardation assays were performed with ≈0.05 pmol of the radiolabeled 270-bp DNA fragment corresponding to the rheA and hsp18 promoters. (A) DNA-binding reactions were carried out at 30°C (lanes 2–4) or 41°C (lanes 5–7) or after a temperature shift-back from 41°C to 30°C (lanes 8 and 9). Quantities of RheA: lane 1, no protein; lanes 2 and 5, 43.2 fmol; lanes 3, 6, and 8, 130 fmol; lanes 4, 7, and 9, 170 fmol. Electrophoresis was at 37°C. (B) RheA was incubated 2 h at 30°C (lanes 2 and 4) or at 41°C (lanes 3 and 5) before a 10-min binding reaction at 30°C. Electrophoresis was at room temperature. Quantities of RheA: lane 1, no protein; lanes 2 and 3, 260 fmol; lanes 4 and 5, 86.5 fmol.