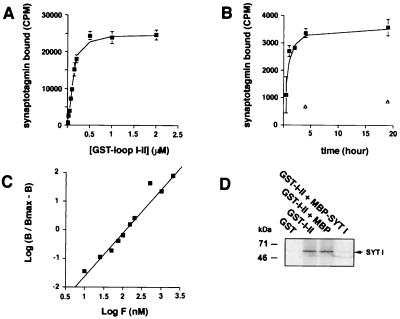
Figure 4.
Characterization of the interaction between synaptotagmin I and αIIA loop I-II. (A) Saturable specific binding of [35S]synaptotagmin I to GST-αIIA. Increasing concentrations of GST-αIIA loop I-II immobilized on glutathione-agarose beads were incubated for 4–5 h at 4°C with a constant amount of [35S]synaptotagmin I. Bound radioactivity was measured by scintillation counting (mean ± SD, n = 3), and nonspecific binding to GST alone was subtracted. Half maximum saturation gave an approximate KD = 100–150 nM. (B) Association kinetics of [35S]synaptotagmin I to GST-αIIA loop I-II (0.05 μM) at 4°C. Maximum binding occurred at 4 h of incubation. Open triangles show nonspecific binding (mean ±SD, n = 3). (C) Hill plot. B, specific binding; Bmax, maximum specific binding; F, GST fusion protein concentration. The slope of the linear regression was 1.6, and the calculated KD was 100nM. (D) [35S]synaptotagmin I (SYT I) was incubated with GST alone or GST-αIIA loop I-II (1 μM) or GST-αIIA loop I-II with MBP-synaptotagmin I or MBP in excess (10 μM). Bound proteins were analyzed by autoradiography.