Figure 2.
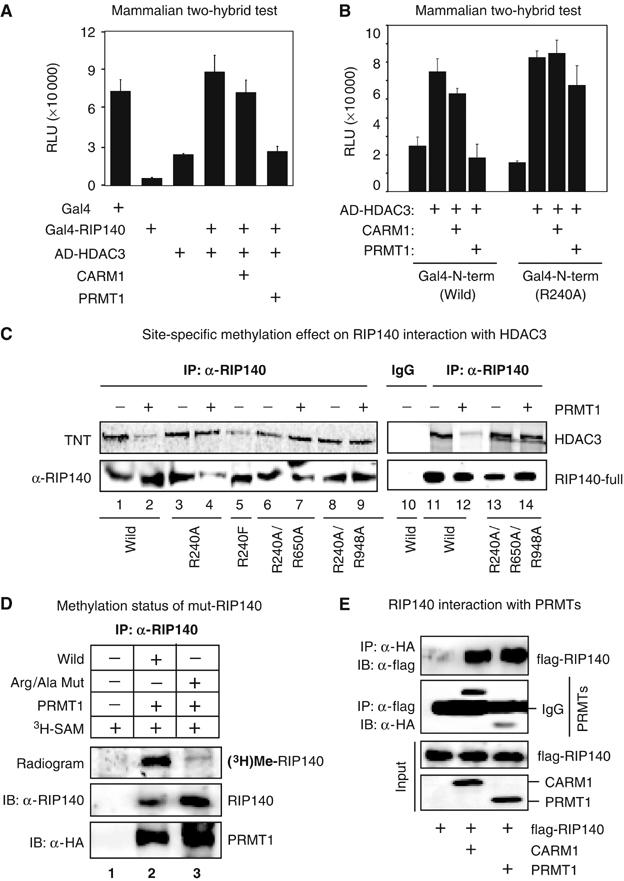
Effect of arginine methylation on the interaction of RIP140 with HDAC3. (A) In a mammalian two-hybrid test on Gal4-Luc reporter in COS-1 cells, Gal4BD-RIP140 (bait) and VP16-ADHADC3 (prey) were expressed alone, or together, in the presence or absence of PRMTs. Hypermethylation induced by PRMT1, but not CARM1, reduced the interaction between RIP140 and HDAC3. (B) In COS-1 cells, hypermethylation reduced the interaction of the wild N-terminal domain (1–495 aa) with HDAC3. The Gal4-R240A mutant of the N-terminal domain constitutively interacted with VP16AD-HDAC3, which was not affected by PRMT1. (C) Hypermethylation induced by PRMT1 in COS-1 cells reduced the direct interaction of RIP140 with in vitro translated HDAC3 as depicted by in vitro IP experiment, whereas the interactions of the Arg/Ala mutants of RIP140 were not affected. (D) Triple-Arg/Ala mutation attenuated the methylation level of RIP140, confirming the specificity of the methylated sites determined from the LC-ESI-MS/MS analysis. (E) In vivo interaction of RIP140 with PRMT1, or CARM1 in a co-IP experiment conducted in COS-1 cells.
