Figure 3.
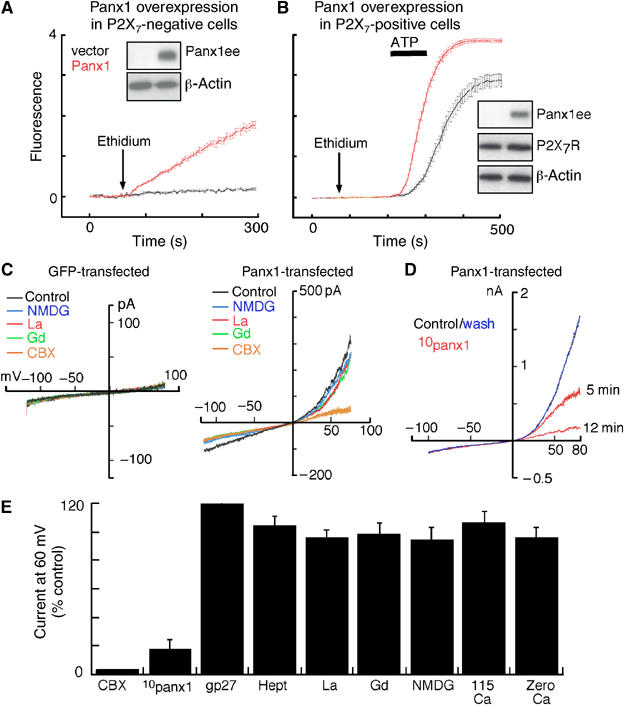
Overexpression of panx1 induces constitutive dye-uptake and hemichannel-like currents selectively blocked by 10panx1 and CBX. (A) Ethidium uptake in P2X7R-negative HEK cells transfected with empty or panx1 expression vector; superfusion of cells with ethidium resulted in immediate uptake in panx1 transfected cells; each trace is mean±s.e.m. of 50 cells in field of view. (B) Similar experiment in P2X7R-positive HEK cells; ethidium application did not result in constitutive dye uptake but the ATP-evoked response showed faster kinetics and maximum fluorescence; traces are mean±s.e.m. of 50 cells. Western blots obtained from cells used in these experiments are shown; panx1 overexpression did not change P2X7R protein expression. (C) Currents in response to ramp voltages (−120 to 80 mV) from control (GFP-transfected) or panx1 transfected HEK cell in normal extracellular solution, in solution containing the larger cation NMDG in place of sodium, in the presence of lanthanum (100 μM), gadolinium (100 μM) or CBX (5 μM) as indicated. (D) Currents from panx1 transfected HEK cell in 0 (control), 5 and 12 min in presence of 100 μM 10panx1 peptide and 15 min after washout; complete inhibition was observed within 10–15 min. (E) Summary of all similar experiments where current at 60 mV is shown as % of control response; only 10panx1 peptide and CBX (20 μM) inhibited the current while other connexin hemichannel inhibitors (heptanol, gp27 peptide and replacing sodium with calcium) or Trp channel inhibitors (NMDG, lanthanum, gadolinium) were without effect. Removal of extracellular calcium, which activates connexin hemichannel currents, also was without effect on panx1 currents (n=24 for CBX and lanthanum, nine for 10panx1 peptide and six for others).
